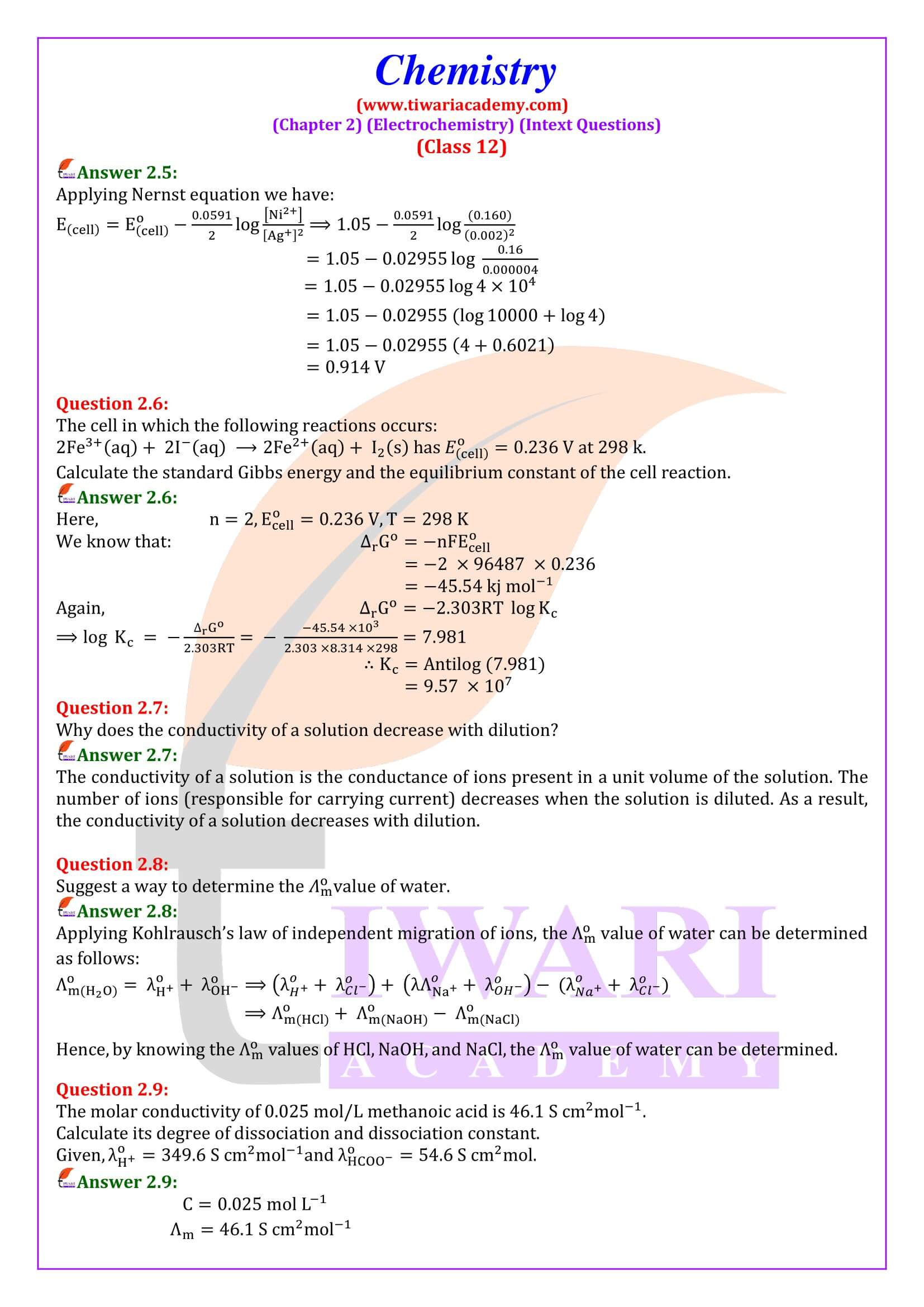
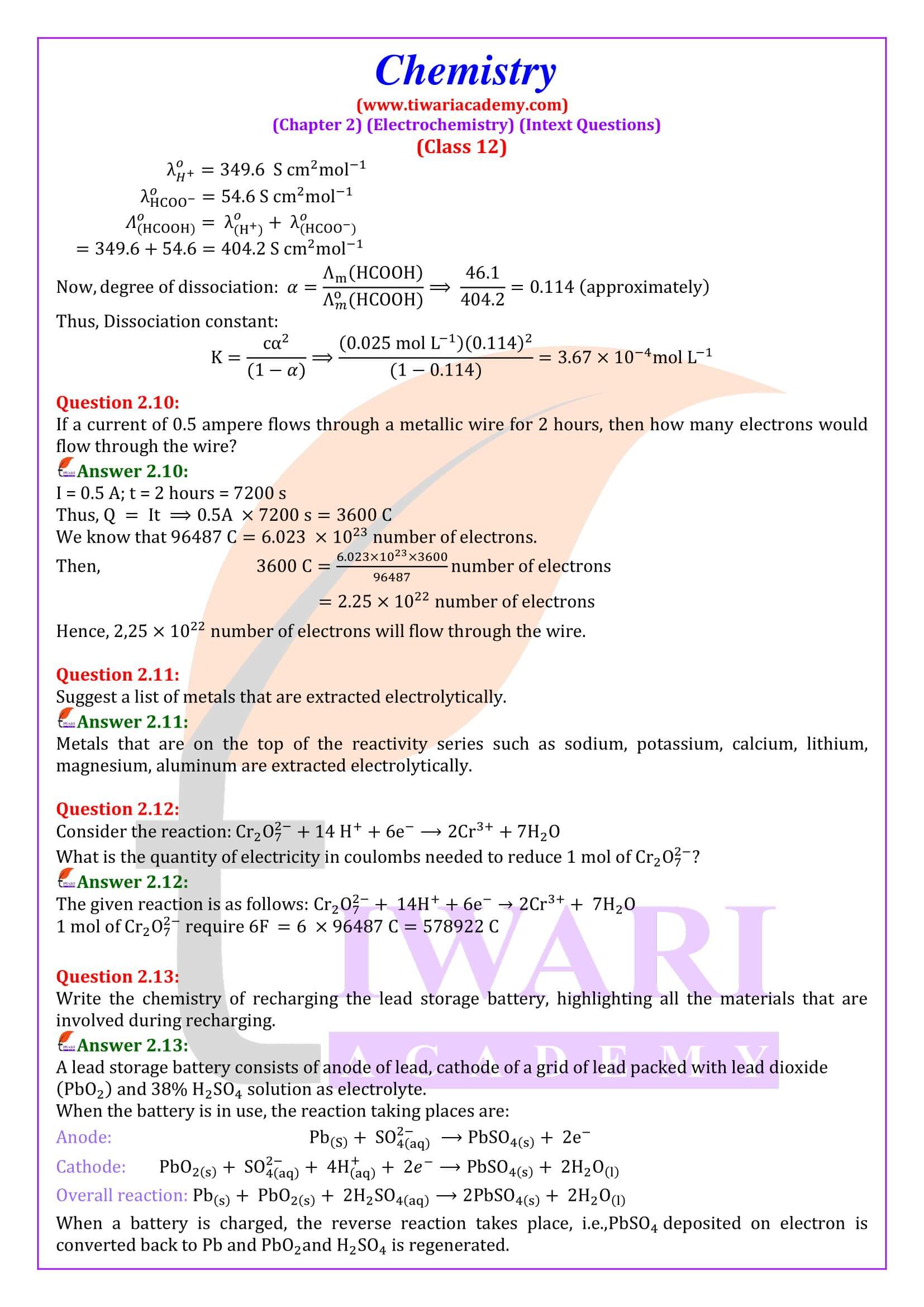
NCERT Solutions for Class 12 Chemistry Chapter 2 Electrochemistry in Hindi Medium and English Medium exercises and intext question answers. These chemistry solutions are useful for CBSE and state board session 2024-25. Class 12 Chemistry unit 2 solutions are useful to understand the concepts of the chapter also using the videos given here.
NCERT Solutions for Class 12 Chemistry Chapter 2
Class 12 Chemistry Chapter 2 Electrochemistry Question Answers

| Class: 12 | Chemistry |
| Chapter: 2 | Electrochemistry |
| Content: | Exercises and Intext Question Answers |
| Mode of Content: | Images, PDF, Text and Videos |
| Academic Session: | 21023-24 |
| Medium: | Hindi and English |
Electrochemistry
Electrochemistry is the study of production of electricity from energy released during spontaneous chemical reactions and the use of electrical energy to bring about non-spontaneous chemical transformations.
Example
Different types of batteries.
Electrochemical Cells
An electrochemical cell is a device or a mechanism that can either generate electrical energy from a chemical reaction or utilize electrical energy to produce a chemical reaction.
Example
A common example of an electrochemical cell is a standard 1.5-volt cell which is used to power many electrical appliances such as TV remotes and clocks. Such cells capable of generating an electric current from the chemical reactions occurring in them care called Galvanic cells or Voltaic cells.
Class 12 Chemistry Chapter 2 MCQ
Fused NaCl on electrolysis gives _____ on cathode.
A conductivity cell containing electrodes made up of
Standard solution of KNO₃ is used to make a salt bridge because
Conductance of Electrolytic Solutions

The conductance of electricity by ions present in solutions is called electrolytic conductance or ionic conductance. Conductivity of electrolytic solution depends on following factors: Nature of electrolyte. Size of ions produced and their solvation. The conductivity of electrolytic (ionic) solutions depends on:
- the nature of the electrolyte added
- size of the ions produced and their solvation
- the nature of the solvent and its viscosity
- concentration of the electrolyte
- temperature
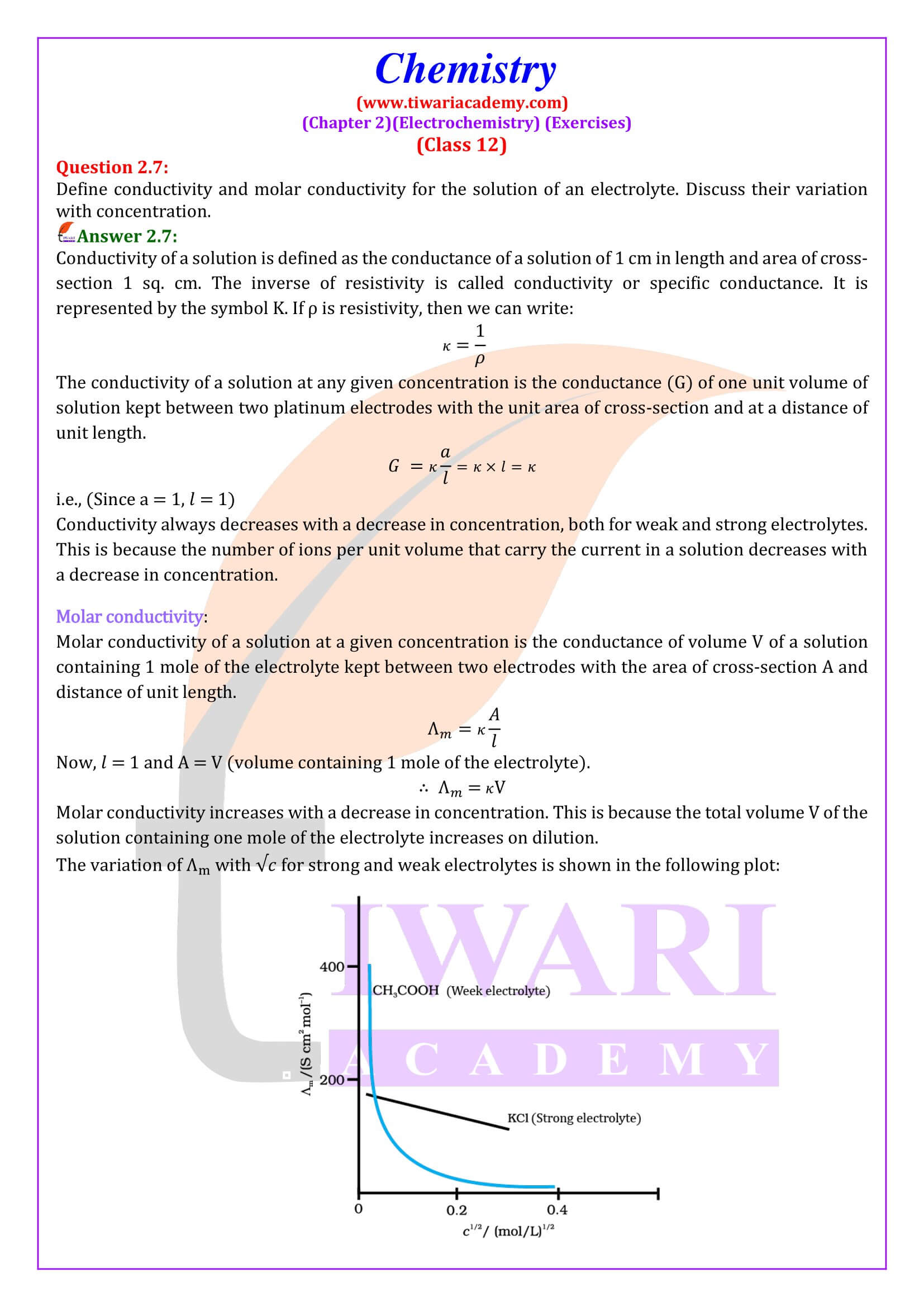
Variation of Conductivity with Concentration
Both conductivity and molar conductivity change with the concentration of the electrolyte. Conductivity always decreases with decrease in concentration both, for weak and strong electrolytes.
Molar Conductivity with Concentration
Molar conductivity of a solution at a given concentration is the conductance of the volume V of solution containing one mole of electrolyte kept between two electrodes with area of cross section A and distance of unit length.
12th Chemistry Unit 2 Multiple Choice Questions
Which of the following is a secondary cell?
While charging the lead storage battery
Electrical conductance through metals is called metallic or electronic conductance and is due to the movement of electrons. The electronic conductance depends on
Rust is a mixture of
Electrolytic Cells
Any device in which electrical energy is converted to chemical energy, or vice versa.
Example
Batteries used in vehicles, UPS, inverters etc.
Electrolysis
electrolysis, process by which electric current is passed through a substance to effect a chemical change. The chemical change is one in which the substance loses or gains an electron (oxidation or reduction).
One of the simplest electrolytic cell consists of two copper strips dipping in an aqueous solution of copper sulphate.
If a DC voltage is applied to the two electrodes, then Cu 2+ ions discharge at the cathode (negatively charged) and the following reaction takes place:
Cu²⁺ (aq) + 2e⁻ ⟶ Cu (s)
Copper metal is deposited on the cathode. At the anode, copper is converted into Cu²⁺ ions by the reaction:
Cu(s) ⟶ Cu²⁺ (s) + 2e⁻
Primary Batteries
Primary batteries are single-use galvanic cells that store electricity for convenient usage, usually showing a good shelf life. Examples are zinc–carbon (Leclanché) cells, alkaline zinc–manganese dioxide cells, and metal–air-depolarized batteries. Primary lithium cells are now available.
Secondary Battery
The storage battery, secondary battery, or charge accumulator is a cell or combination of cells in which the cell reactions are reversible. This means that the original chemical conditions within the cell can be restored by passing current to flow into it: that is, by charging from an external source.
Class 12 Chemistry Chapter 2 Important Questions
Rusting of iron is quicker in saline water than in ordinary water.
Electrolytes present in sea water favour the formation of more electrochemical cells on the surface of iron leading to increase in the rate of rusting.
Define limiting molar conductivity. Why conductivity of an electrolyte solution decreases with the decrease in concentration?
Limiting molar conductivity is the maximum conductivity when solution is infinitely dilute, such that on further dilution there is no increase in Am. Conductivity decreases with decrease in concentration because number of ions per unit volume decrease.
What is role of ZnCl₂ in a dry cell?
ZnCl₂ combines with NH₃ produced to form the complex [Zn(NH₃)₂ Cl₂], otherwise the pressure developed due to NH₃ would crack the seal of the cell.