NCERT Solutions for Class 11 Chemistry Chapter 8 Organic Chemistry Some Basic Principles and Techniques updated in Hindi and English Medium for CBSE session 2024-25. Class 11 Chemistry Chapter 8 Exercises, Intext Questions, MCQ and Extra Important Questions Answers and solutions are given here in the format of PDF and Videos.
NCERT Solutions for Class 11 Chemistry Chapter 8
- Class 11 Chemistry Chapter 8 NCERT Solutions
- Class 11 Chemistry Chapter 8 NCERT Book
- Class 11 Chemistry Chapter 8 Hindi Medium
- Class 11 Chemistry Chapter 8 Revision Book
- Class 11 Chemistry Chapter 8 Study Material 1
- Class 11 Chemistry Chapter 8 Study Material 2
- Class 11 Chemistry Chapter 8 Assignment
- Class 11 Chemistry NCERT Solutions
- Class 11 all Subjects NCERT Solutions
Organic Chemistry
carbon has the unique property called catenation due to which it forms covalent bonds with other carbon atoms. It also forms covalent bonds with atoms of other elements like hydrogen, oxygen, nitrogen, sulphur, phosphorus and halogens. The resulting compounds are studied under a separate branch of chemistry called organic chemistry.
Class 11 Chemistry Chapter 8 MCQ
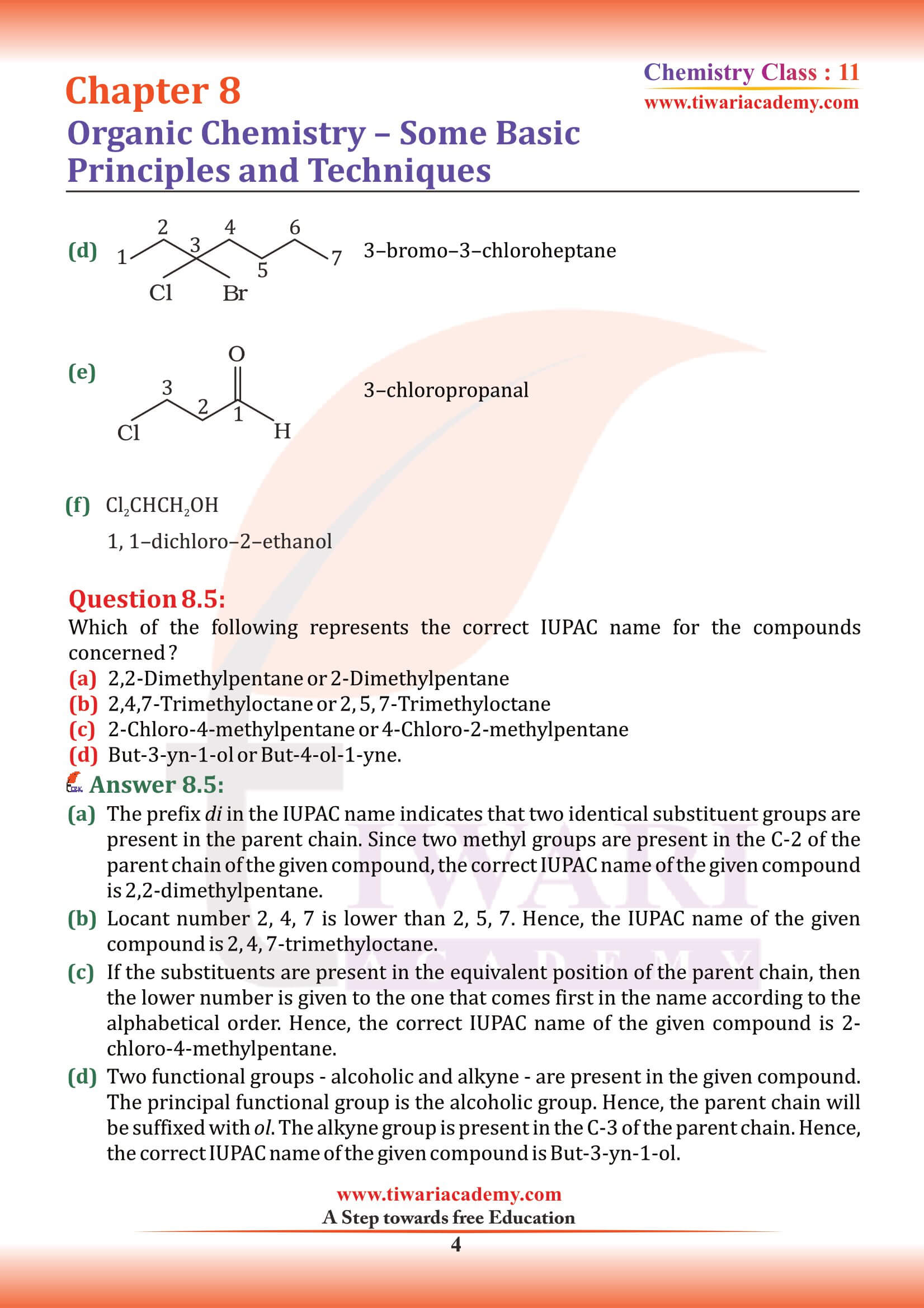
Which of the following is the correct IUPAC name?
Electronegativity of carbon atoms depends upon their state of hybridisation. In which of the following compounds, the carbon marked with asterisk is most electronegative?
In which of the following, functional group isomerism is not possible?
The fragrance of flowers is due to the presence of some steam volatile organic compounds called essential oils. These are generally insoluble in water at room temperature but are miscible with water vapour in vapour phase. A suitable method for the extraction of these oils from the flowers is
Tetravalence of Carbon: Shapes of Organic Compounds
fundamental concepts of molecular structure help in understanding and predicting the properties of organic compounds. tetravalence of carbon and the formation of covalent bonds by it are explained in terms of its electronic configuration and the hybridisation of s and p orbitals. It may be recalled that formation and the shapes of molecules like methane (CH₄), ethene (C₂H₄), ethyne (C₂H₂) are explained in terms of the use of sp³, sp² and sp hybrid orbitals by carbon atoms in the respective molecules.
Structural Representations of Organic Compounds
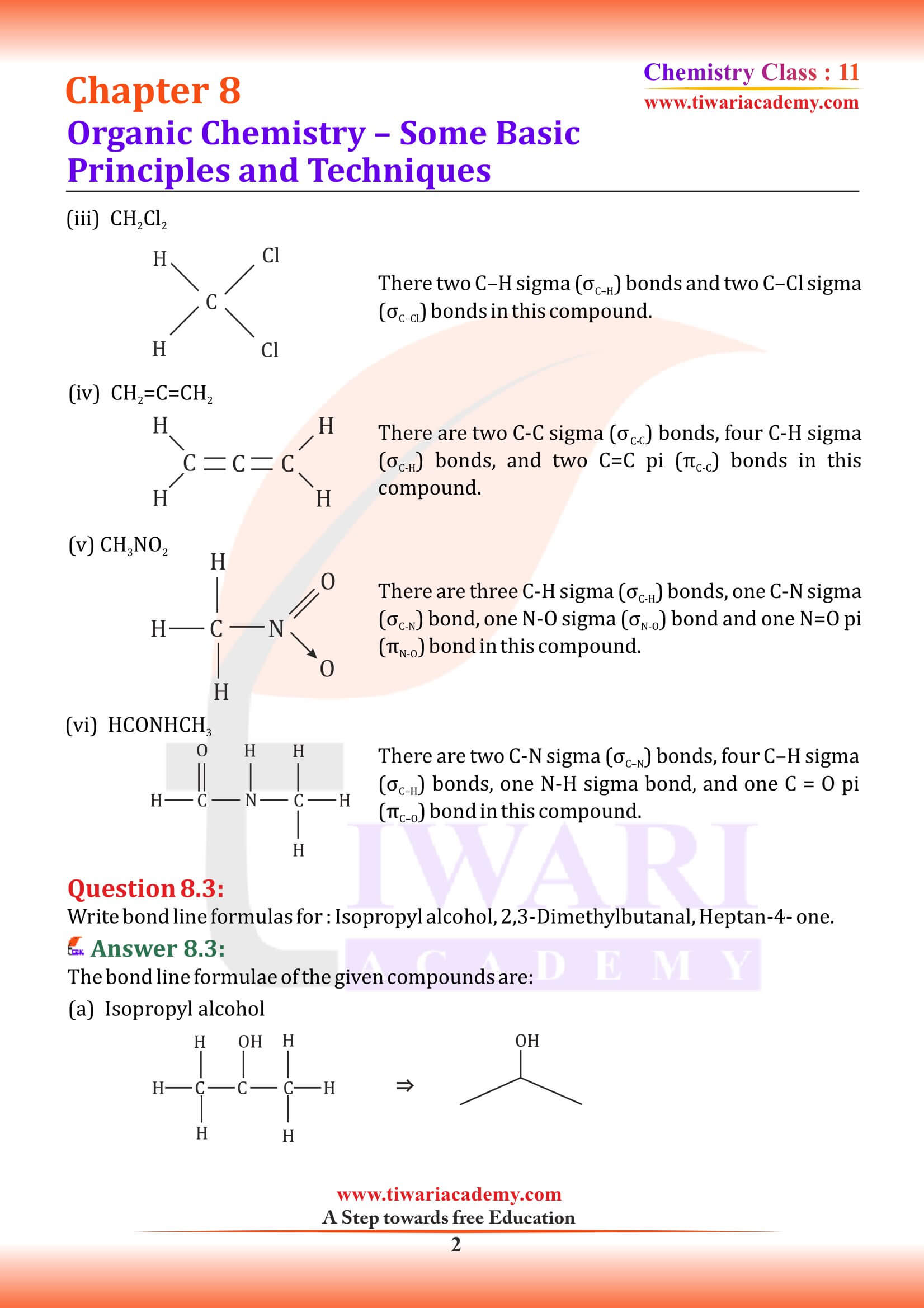
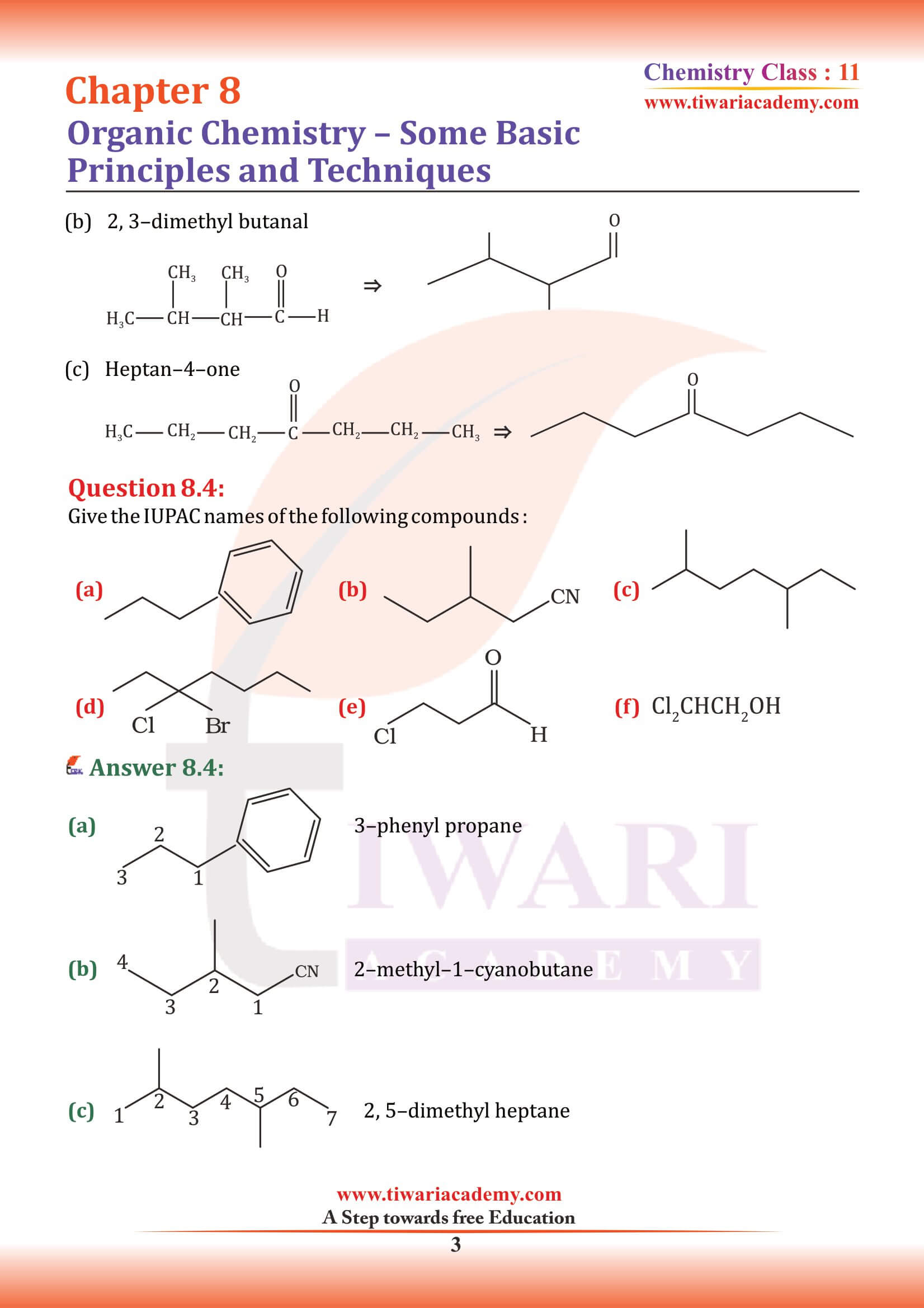
Structures of organic compounds are represented in several ways. The Lewis structure or dot structure, dash structure, condensed structure and bond line structural formulas are some of the specific types. The Lewis structures, however, can be simplified by representing the two-electron covalent bond by a dash (–). Such a structural formula focuses on the electrons involved in bond formation. A single dash represents a single bond, double dash is used for double bond and a triple dash represents triple bond.
Class 11 Chemistry Chapter 8 MCQ with Answers
During hearing of a court case, the judge suspected that some changes in the documents had been carried out. He asked the forensic department to check the ink used at two different places. According to you which technique can give the best results?
The principle involved in paper chromatography is
In which of the following compounds the carbon marked with asterisk is expected to have greatest positive charge?
Nucleophile is a species that should have
Classification of Organic Compounds
Organic compounds are broadly classified as follows:
1. Acyclic or open chain compounds
2. Cyclic or closed chain or ring compounds
Acyclic or Open Chain Compounds
These compounds are also called as aliphatic compounds and consist of straight or branched chain compounds, for example: Ethane (CH₃CH₃), Isobutane {CH₃CH(CH₃)₂}, Acetic acid (CH₃COOH).
Cyclic or Closed Chain or Ring Compounds
Cyclic or closed chain compounds are further classified as:
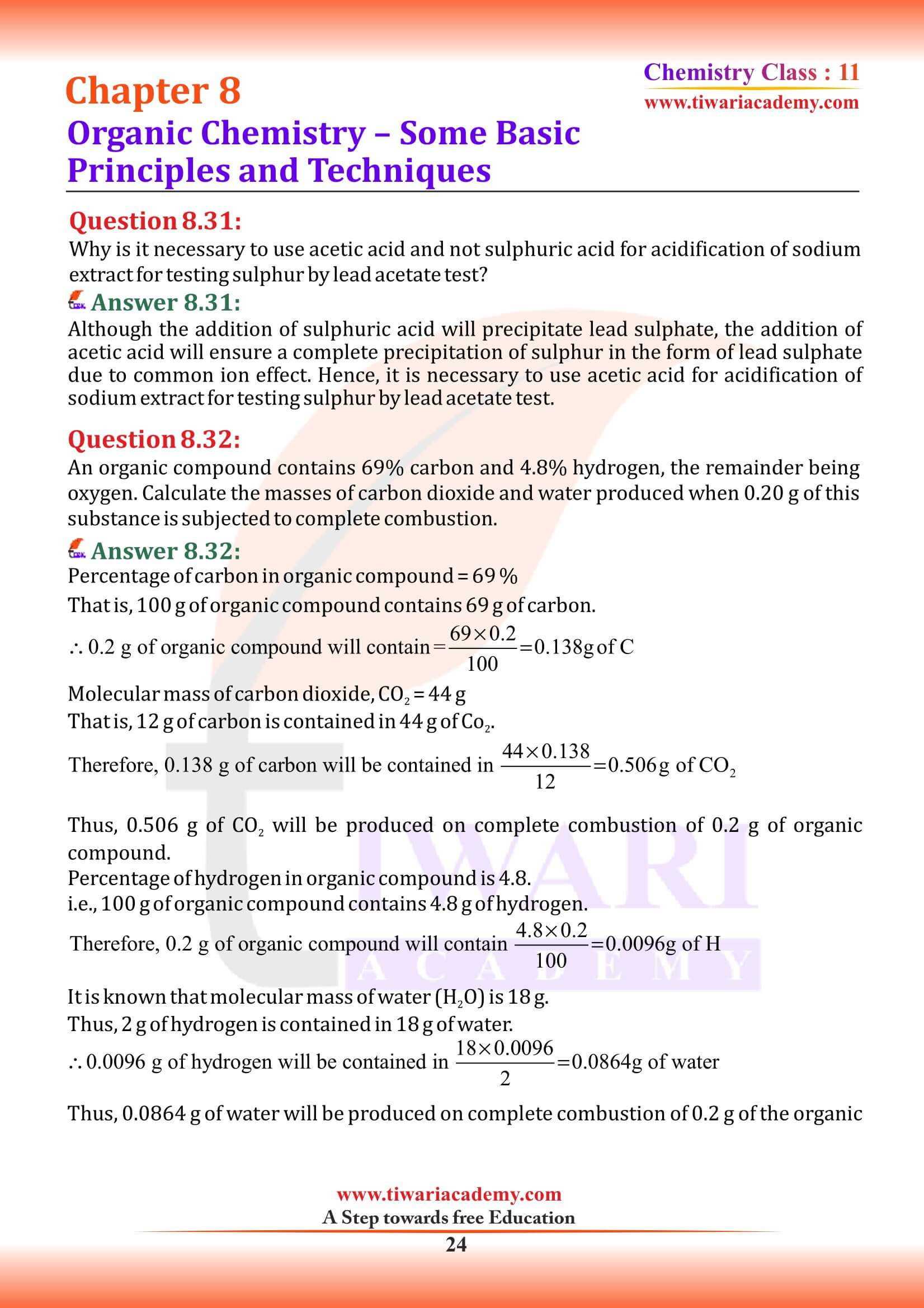
(a) Alicyclic compounds
Alicyclic (aliphatic cyclic) compounds contain carbon atoms joined in the form of a ring, Cyclopropane, Cyclohexane, Cyclohexene are the examples of homocyclic. Sometimes atoms other than carbon are also present in the ring (heterocylic). Tetrahydrofuran given below is an example of this type of compound.
(b) Aromatic compounds
Aromatic compounds are special types of compounds.These include benzene and other related ring compounds (benzenoid). Like alicyclic compounds, aromatic comounds may also have hetero atom in the ring. Such compounds are called hetrocyclic aromatic compounds. Some of the examples of various types of aromatic compounds are: Benzenoid aromatic compounds: Benzene, Aniline, Naphthalene.
Non-benzenoid compound: Tropone.
Heterocyclic aromatic compounds: Furan, Thiophene, Pyridine.
Class 11 Chemistry Chapter 8 Important Extra Questions
Electronegativity increases with increasing s character. sp³ < sp² < sp.
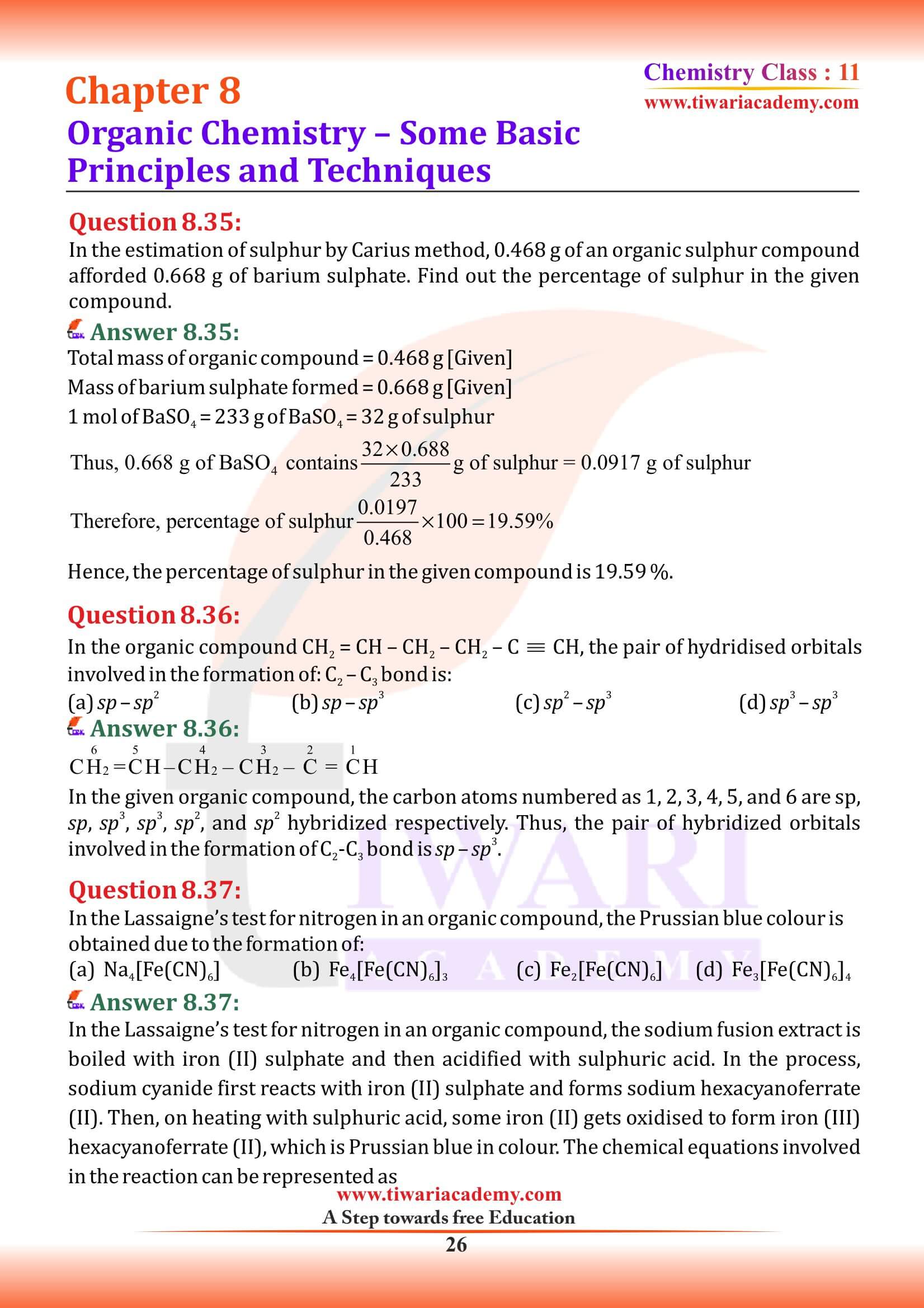
In DNA and RNA, nitrogen atom is present in the ring system. Can Kjeldahl method be used for the estimation of nitrogen present in these? Give reasons.
DNA and RNA have nitrogen in the heterocyclic rings. Nitrogen present in rings, azo groups and nitro groups cannot be removed as ammonia.
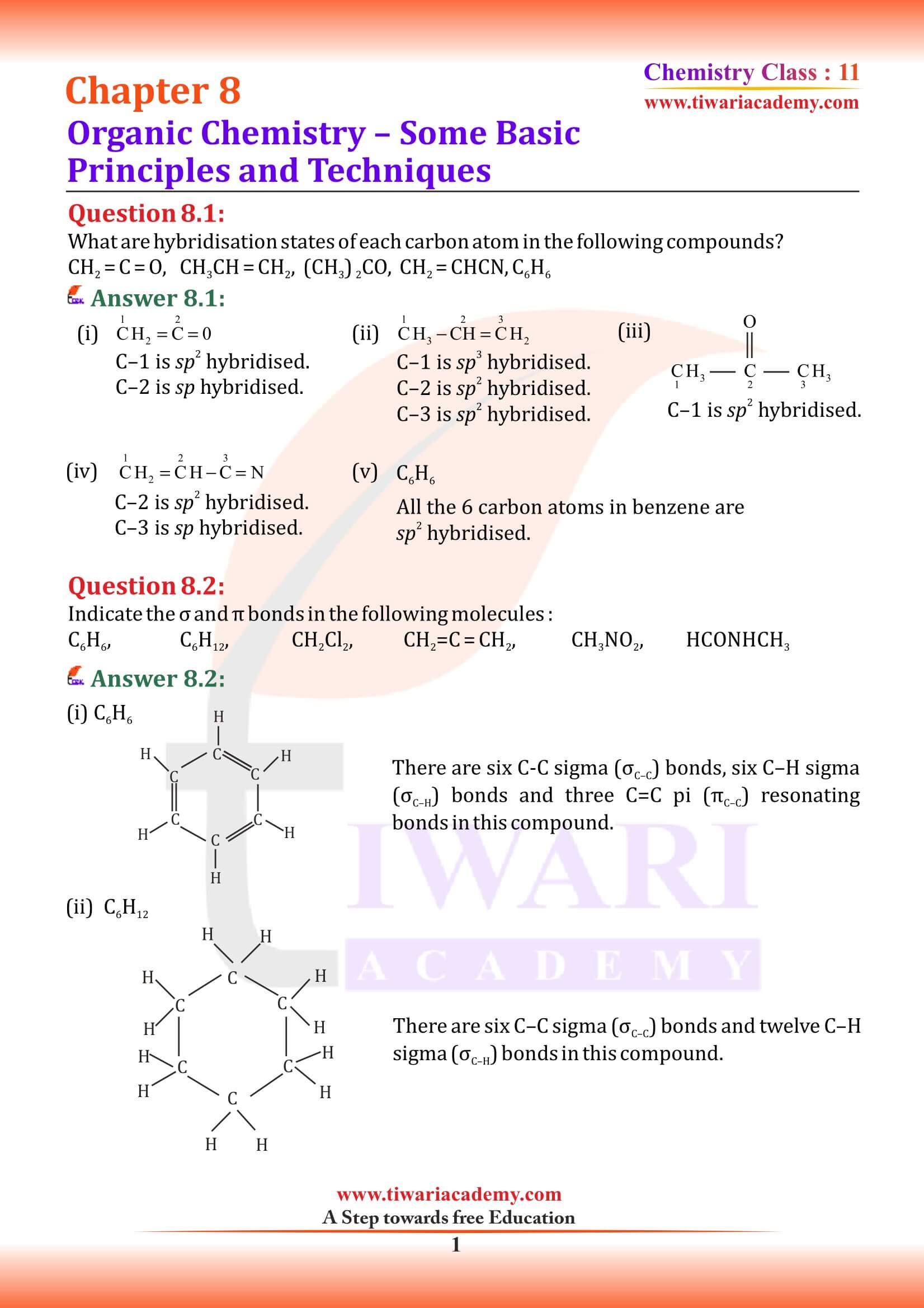
What is the hybridisation of each carbon in H₂C = C = CH₂?
Central atom forms two σ and two π bonds. It consists of sp−sp overlap between two carbon atoms forming two σ bonds. Two additional pi bonds also formed between two carbons atoms (two neighbour carbons). Hence, central carbon atom has sp hybridization.
Isomerism
The phenomenon of existence of two or more compounds possessing the same molecular formula but different properties is known as isomerism. Such compounds are called as isomers.
Methods of Purification of Organic Compounds
Once an organic compound is extracted from a natural source or synthesised in the laboratory, it is essential to purify it. Various methods used for the purification of organic compounds are based on the nature of the compound and the impurity present in it. The common techniques used for purification are as follows:
- (i) Sublimation
- (ii) Crystallisation
- (iii) Distillation
- (iv) Differential extraction and
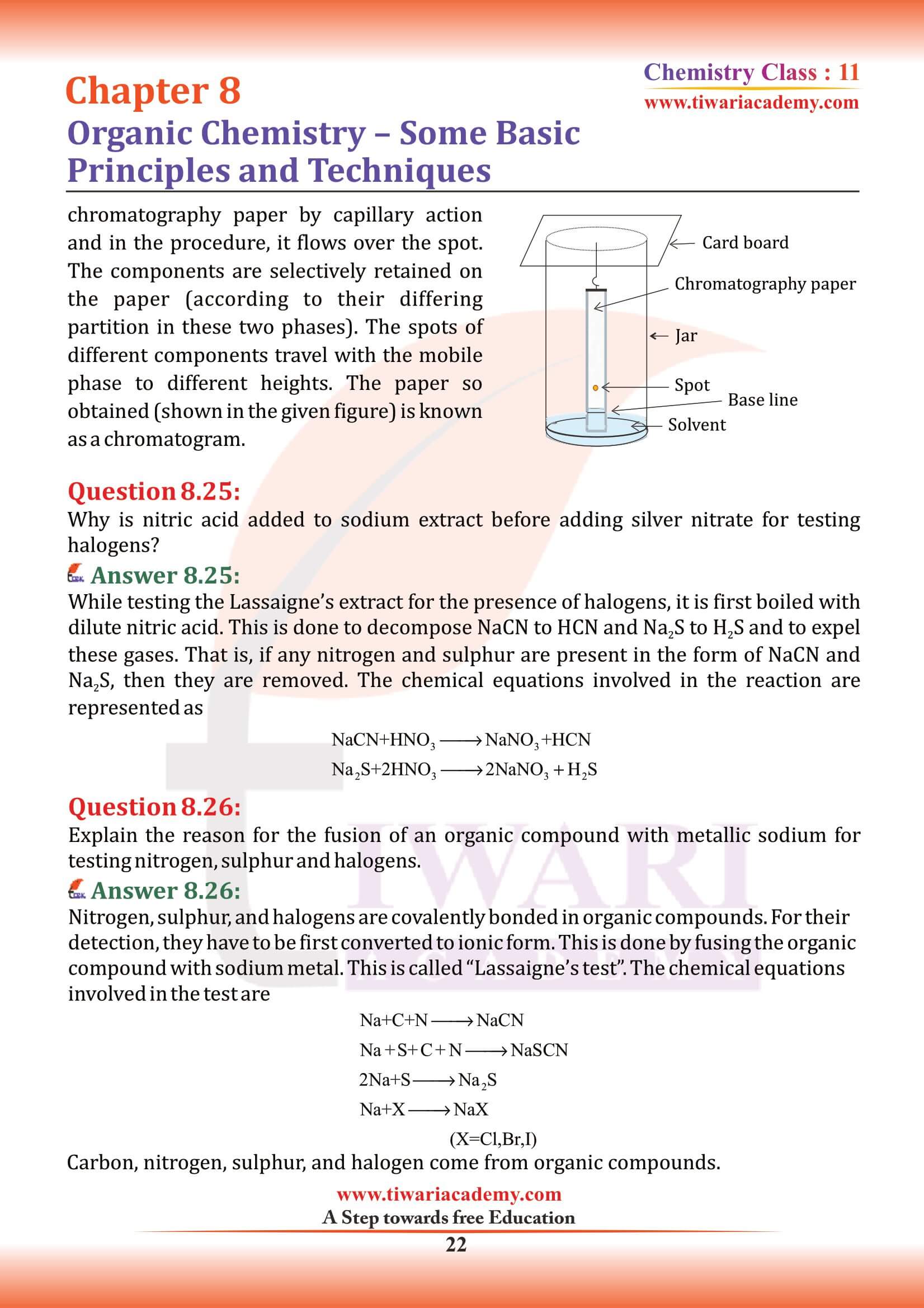
- (v) Chromatography