NCERT Solutions for Class 7 Science Curiosity Chapter 5 Changes Around Us Physical and Chemical for Session 2025-26. NCERT 7th Science Solutions describes clear, well-structured answers to all textbook questions. They help students understand the key differences between physical and chemical changes through real-life examples, activities and scientific explanations. They enhance conceptual clarity, making it easier for students to prepare for exams and apply scientific reasoning in everyday life.
Class 7 Science Solutions
Class 7 Science Curiosity Chapter 5 Solutions
Let Us Enhance Our Learning
1. Which of the following statements are the characteristics of a physical change?
(i) The state of the substance may or may not change.
(ii) A substance with different properties is formed.
(iii) No new substance is formed.
(iv) The substance undergoes a chemical reaction.
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iii)
(d) (iii) and (iv)
See Answer(c) (i) and (iii). (Physical changes involve changes in physical properties like shape, size, or state, but no new substance is formed.)
2. Predict which of the following changes can be reversed and which cannot be reversed. If you are not sure, you may write that down. Why are you not sure about these?
(i) Stitching cloth to a shirt
(ii) Twisting of straight string
(iii) Making idlis from a batter
(iv) Dissolving sugar in water
(v) Drawing water from a well
(vi) Ripening of fruits
(vii) Boiling water in an open pan
(viii) Rolling up a mat
(ix) Grinding wheat grains to flour
(x) Forming of soil from rocks
See Answer(i) Stitching cloth to a shirt: Cannot be reversed (easily). (Cutting/stitching alters original cloth permanently).
(ii) Twisting of straight string: Can be reversed.
(iii) Making idlis from a batter: Cannot be reversed. (Chemical changes during cooking).
(iv) Dissolving sugar in water: Can be reversed (by evaporating the water).
(v) Drawing water from a well: Can be reversed (physical change of location).
(vi) Ripening of fruits: Cannot be reversed (chemical changes).
(vii) Boiling water in an open pan: Can be reversed (water evaporates, can be condensed).
(viii) Rolling up a mat: Can be reversed.
(ix) Grinding wheat grains to flour: Cannot be reversed (practically). (Physical change, but difficult to reassemble grains).
(x) Forming of soil from rocks: Cannot be reversed. (Very slow physical and chemical changes over long periods).
3. State whether the following statements are True or False. In case a statement is False, write the correct statement.
(i) Melting of wax is necessary for burning a candle. (True/False)
(ii) Collecting water vapour by condensing involves a chemical change. (True/False)
(iii) The process of converting leaves into compost is a chemical change. (True/False)
(iv) Mixing baking soda with lemon juice is a chemical change. (True/False)
See Answer(i) True. (Wax melts, evaporates and the vapour burns).
(ii) False. Correct statement: Collecting water vapour by condensing involves a physical change (change of state from gas to liquid).
(iii) True. (Decomposition involves chemical reactions).
(iv) True. (Produces carbon dioxide gas and other new substances).
4. Fill in the blanks in the following statements:
(i) Nalini observed that the handle of her cycle has got brown deposits. The brown deposits are due to ______ and this is a ______ change.
(ii) Folding a handkerchief is a ______ change and can be ______.
(iii) A chemical process in which a substance reacts with oxygen with evolution of heat is called ______ and this is a ______ change.
(iv) Magnesium, when burnt in air, produces a substance called ______. The substance formed is ______ in nature. Burning of magnesium is a ______ change.
See Answer(i) The brown deposits are due to rusting and this is a chemical change.
(ii) Folding a handkerchief is a physical change and can be reversed.
(iii) A chemical process in which a substance reacts with oxygen with evolution of heat is called combustion and this is a chemical change.
(iv) Magnesium, when burnt in air, produces a substance called magnesium oxide. The substance formed is basic in nature. Burning of magnesium is a chemical change.
5. Are the changes of water to ice and water to steam, physical or chemical? Explain.
See AnswerThese are physical changes.
Explanation: In both cases (freezing water to ice, boiling water to steam), only the physical state of water changes (liquid to solid, liquid to gas). The chemical substance remains water (H₂O). No new substance with different chemical properties is formed and these changes are readily reversible.
6. Is curdling of milk a physical or chemical change? Justify your statement.
See AnswerCurdling of milk is a chemical change.
Justification: Milk turns into curd, which is a new substance with different properties (taste, texture, chemical composition). This change involves chemical reactions (fermentation) and is irreversible.
7. Natural factors, such as wind, rain, etc., help in the formation of soil from rocks. Is this change physical or chemical and why?
See AnswerThe formation of soil from rocks involves both physical and chemical changes.
Why: Rocks break down into smaller pieces due to temperature changes, freezing water or plant roots, which are physical changes. Rocks can also react chemically with water, air (oxygen) or acids dissolved in rainwater, changing their composition (like rusting of iron minerals in rocks), which are chemical changes. The overall process is called weathering.
8. Read the following story titled ‘Eco-friendly Prithvi’, and tick the most appropriate option(s) given in the brackets. Provide a suitable title of your choice for the story.
Prithvi is preparing a meal in the kitchen. He chops vegetables, peels potatoes, and cuts fruits (physical changes/chemical changes). He collects the seeds, fruits, and vegetable peels into a clay pot (physical change/chemical change). The fruits, vegetable peels, and other materials begin to decompose due to the action of bacteria and fungi, forming compost (physical change/chemical change). He decides to plant seeds in the compost and water them regularly. After a few days, he notices that the seeds begin to germinate and small plants start to grow, eventually blooming into colourful flowers (physical change/chemical change). His efforts are appreciated by all his family members.
See AnswerHe chops vegetables, peels potatoes, and cuts fruits (physical changes).
He collects the seeds, fruits and vegetable peels into a clay pot (physical change).
The fruits, vegetable peels and other materials begin to decompose… forming compost (chemical change).
…seeds begin to germinate and small plants start to grow, eventually blooming into colourful flowers (chemical changes – involving complex life processes).
Suitable Title: (Examples) “Prithvi’s Compost Garden”, “Waste to Flowers”, “Eco-friendly Gardening”.
9. Some changes are given here. Write physical changes in the area marked ‘A’ and chemical changes in the area marked ‘B’. Enter the changes which are both physical and chemical in the area marked ‘C’. (Venn Diagram)
Process of burning a candle; Tearing of paper; Rusting; Curdling of milk; Ripening of fruits; Melting of ice; Folding of clothes; Burning of magnesium and Mixing baking soda with vinegar.
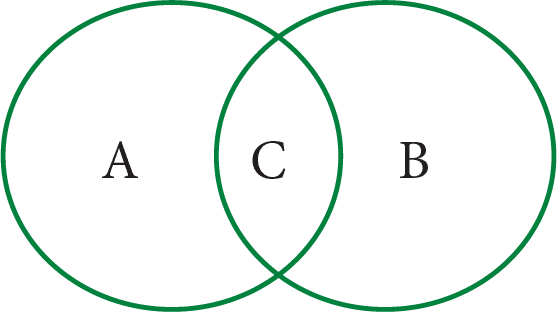
See AnswerA (Physical Changes only): Tearing of paper, Melting of ice, Folding of clothes.
B (Chemical Changes only): Rusting, Curdling of milk, Ripening of fruits, Burning of magnesium, Mixing baking soda with vinegar.
C (Both Physical and Chemical): Process of burning a candle.
10. The experiments shown in Fig. 5.11a, b, c and d were performed. Find out in which case(s) did lime water turn milky and why? (Diagram shows reactions piped into lime water)
(a) Vinegar and baking soda
(b) Lemon juice and vinegar
(c) Vinegar and common salt
(d) Lemon juice and baking soda
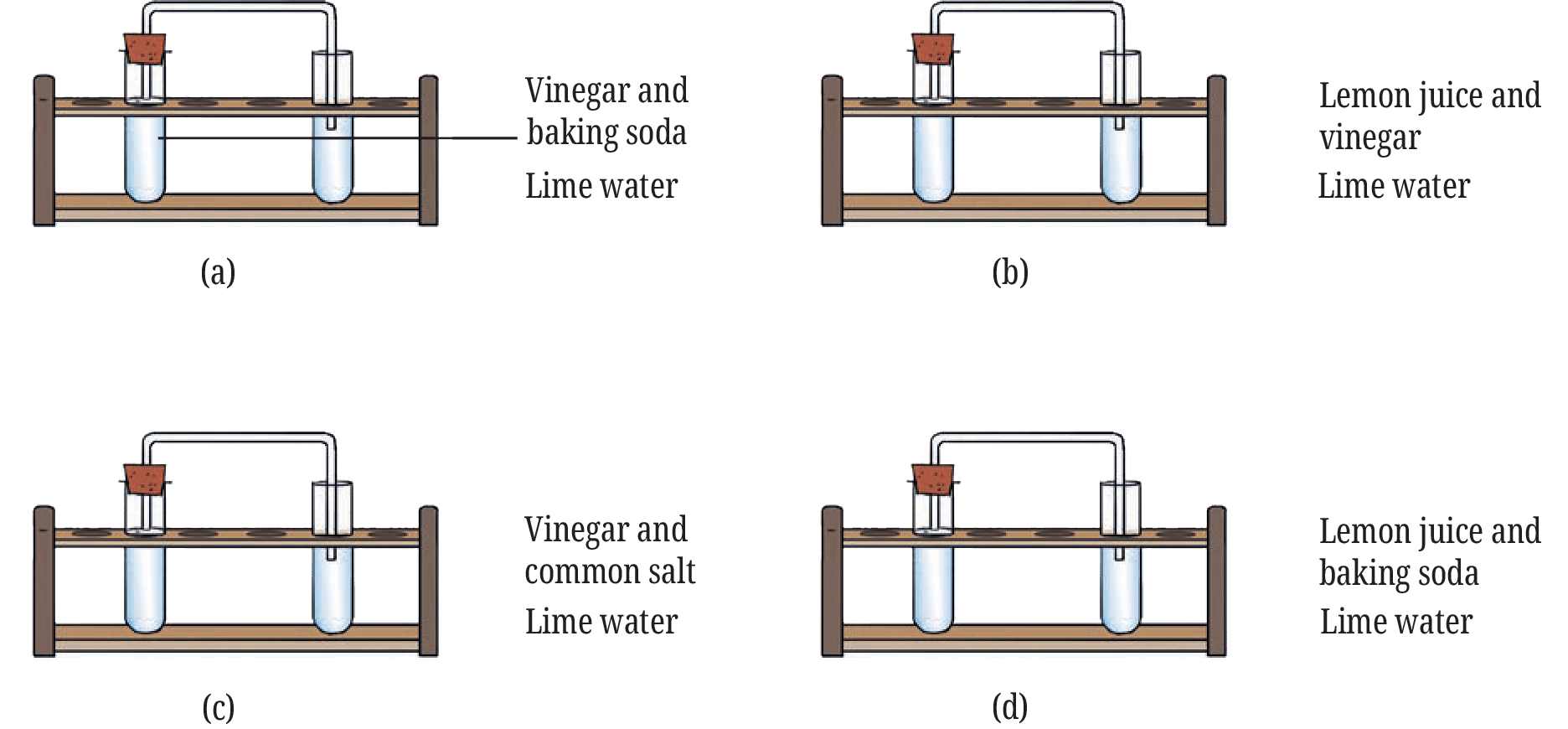
See AnswerLime water turned milky in cases (a) and (d).
Why: Lime water turns milky in the presence of carbon dioxide gas. The reaction between an acid (vinegar or lemon juice) and baking soda produces carbon dioxide gas. In cases (b) and (c), mixing two acids or an acid and common salt does not typically produce carbon dioxide.
Class 7 Science Curiosity Chapter 5 Very Short Answer Type Questions
What is a physical change?
See AnswerA physical change is one in which only the appearance, state, or size of a substance changes and no new substance is formed.
What is a chemical change?
See AnswerA chemical change is one in which a new substance is formed as a result of a chemical reaction.
Give one example of a reversible change.
See AnswerMelting of ice is a reversible change, as the water can be frozen again to form ice.
Which gas turns lime water milky?
See AnswerCarbon dioxide gas turns lime water milky, indicating a chemical change.
What is ignition temperature?
See AnswerIt is the minimum temperature at which a substance catches fire and starts to burn.
Class 7 Science Curiosity Chapter 5 Short Answer Type Questions
How is a physical change different from a chemical change?
See AnswerA physical change affects only the physical properties and is usually reversible. A chemical change produces new substances and is mostly irreversible.
Why is rusting of iron considered a chemical change?
See AnswerRusting of iron forms a new substance called iron oxide when iron reacts with air and moisture. Since a new substance is formed, it is a chemical change.
What is combustion?
See AnswerCombustion is a chemical reaction where a substance reacts with oxygen to release heat and/or light. Examples include burning wood, kerosene, and magnesium.
How does burning of a candle involve both physical and chemical changes?
See AnswerMelting and solidifying of wax are physical changes, while burning of wax vapour that produces new substances is a chemical change.
What is the role of oxygen in combustion?
See AnswerOxygen supports combustion. Without it, a substance cannot burn, as shown in the experiment where a candle goes out when covered with a glass.
Class 7 Science Curiosity Chapter 5 Descriptive Answer Type Questions
What are the three essential conditions for combustion?
See AnswerCombustion requires:
1. A combustible substance (fuel),
2. Oxygen (from air) and
3. Heat (to raise the fuel to its ignition temperature).
All three are needed for a fire to start and continue. Removing any one of these will stop the combustion.
Describe what happens when vinegar reacts with baking soda.
See AnswerWhen vinegar is mixed with baking soda, a chemical reaction occurs producing bubbles of carbon dioxide gas. This gas turns lime water milky, confirming a new substance has formed—proving it is a chemical change.
Why is burning of magnesium a chemical change?
See AnswerBurning magnesium in air forms a new white substance called magnesium oxide, along with light and heat. Since a new substance is produced, it is a chemical change.
How do both physical and chemical changes occur in a burning candle?
See AnswerIn a burning candle, the wax melts (physical change), vaporises, and then burns to form new substances (chemical change). Thus, both types of changes occur simultaneously.
What is weathering of rocks? Explain with an example.
See AnswerWeathering is the gradual breaking down of rocks into soil by physical (temperature, wind, water) and chemical processes. For example, black basalt rock turns red due to chemical reaction with water and air, forming iron oxide.
Class 7 Science Curiosity Chapter 5 Exploring Questions
Why are some changes considered undesirable?
See AnswerSome changes cause harm or damage. Rusting of iron weakens structures, and food spoilage makes it unsafe to eat. However, some changes like composting waste may seem undesirable but are beneficial in the right context.
Can chemical changes be prevented? Explain with an example.
See AnswerYes. Chemical changes like rusting can be prevented using methods such as painting, oiling or galvanising to protect iron from moisture and air, which trigger rust.
Why is burning paper using sunlight a chemical change?
See AnswerWhen paper is focused on with sunrays using a magnifying glass, it catches fire and turns into ash and gases—new substances. This irreversible process makes it a chemical change.
Is curdling of milk a physical or chemical change? Justify.
See AnswerCurdling of milk is a chemical change. Bacteria convert the milk into curd by changing its proteins and acids. A new substance is formed and the change cannot be reversed.
How does erosion represent a physical change?
See AnswerErosion involves the physical breaking and transportation of rocks and soil by wind or water. No new substance is formed and only the form or location changes, making it a physical change.
Why do some changes around us happen quickly while others take years, as explained in Class 7 Science Curiosity Chapter 5?
According to Class 7 Science Curiosity Chapter 5, changes in our surroundings occur at different speeds based on the type of process involved. Some changes, like melting of ice, boiling of water, or burning a candle, happen within seconds or minutes. These are usually physical or fast chemical changes. On the other hand, changes such as weathering of rocks, formation of soil, or rusting of iron take days, years, or even centuries. Weathering and erosion are slow processes influenced by natural forces like rain, wind and temperature changes. The speed of a change depends on factors like the substance involved, temperature and environmental conditions.
Can one activity involve both physical and chemical changes at the same time in Class 7 Science Curiosity Chapter 5?
Yes, Class 7 Science Curiosity Chapter 5 explains that a single activity can involve both physical and chemical changes. A great example is the burning of a candle. The melting of wax, its evaporation, and solidifying into a new shape are all physical changes because no new substance is formed and these can be reversed. However, the wax vapour burns and reacts with oxygen in the air to form new substances like carbon dioxide and water, producing light and heat—this is a chemical change. This example helps students understand that real-life processes are often complex and not limited to one type of change.
How can we test for the presence of carbon dioxide gas using a simple classroom method, as taught in Class 7 Science Curiosity Chapter 5?
Class 7 Science Curiosity Chapter 5 introduces a simple and effective way to test for carbon dioxide gas using lime water. When carbon dioxide gas is passed through lime water, it reacts to form calcium carbonate, which turns the lime water milky or cloudy. This is a sign that a chemical reaction has occurred. One common classroom activity uses vinegar and baking soda to produce carbon dioxide. The gas is then directed into a test tube containing lime water, which turns milky, confirming the gas’s presence. This experiment not only shows a chemical change but also teaches students how to identify gases using visible clues.
What types of changes do I need to remember in Class 7 Science Curiosity Chapter 5?
In Chapter 5, focus on two main types of changes: physical changes and chemical changes. Physical changes don’t form new substances and are usually reversible—like melting ice. Chemical changes create a new substance and are mostly irreversible—like rusting or curdling milk. Remembering a few everyday examples of each makes it easier to identify and answer questions quickly.
Is Class 7 Science Curiosity Chapter 5 difficult to understand?
Not at all! Chapter 5 is quite easy and interesting because it’s all around you. Boiling water, burning paper, curdling milk or rusting nails are examples you see every day. If you focus on understanding the difference between temporary and permanent changes and relate them to your daily life, it becomes one of the simplest chapters in the book.
How can I prepare for Class 7 Science Curiosity Chapter 5 in a better way?
To prepare well, make a T-chart of physical vs. chemical changes with examples. Try the simple experiments from the book—like mixing vinegar and baking soda or testing lime water with CO₂. Also, revise definitions and practice distinguishing between reversible and irreversible changes. Watching short science videos or explaining the concepts to a friend can help you remember them better.


