NCERT Solutions for Class 8 Science Curiosity Chapter 8 Nature of Matter: Elements, Compounds and Mixtures for Session 2025-26. Grade 8 Science Solutions provide a clear explanation of the basic building blocks of matter. The chapter helps students differentiate between elements, compounds and mixtures through examples and activities. It explains their properties, uses and significance in daily life. These solutions encourage conceptual clarity, analytical thinking, and application-based learning. With step-by-step answers, they make understanding scientific concepts easier and prepare students for higher-level studies.
Class 8 Science Curiosity Chapter 8 MCQ
Class 8 Science Old Book Chapter 8 MCQ
Class 8 Science Old Book Chapter 8 Solutions
Nature of Matter: Elements, Compounds and Mixtures Class 8 Science Curiosity Chapter 8 Answers
1. Consider the following reaction where two substances, A and B, combine to form a product C:
A + B → C
Assume that A and B cannot be broken down into simpler substances by chemical reactions. Based on this information, which of the following statements is correct?
(i) A, B, and C are all compounds and only C has a fixed composition.
(ii) C is a compound, and A and B have a fixed composition.
(iii) A and B are compounds, and C has a fixed composition.
(iv) A and B are elements, C is a compound, and has a fixed composition.
See Answer(iv) A and B are elements, C is a compound and has a fixed composition.
2. Assertion: Air is a mixture.
Reason: A mixture is formed when two or more substances are mixed, without undergoing any chemical change.
(i) Both Assertion and Reason are true and Reason is the correct explanation for Assertion.
(ii) Both Assertion and Reason are true, but Reason is not the correct explanation for Assertion.
(iii) Assertion is true, but Reason is false.
(iv) Assertion is false, but Reason is true.
See Answer(i) Both Assertion and Reason are true and Reason is the correct explanation for Assertion.
Class 8 Science Curiosity Chapter 8 Question 3, 4 and 5
3. Water, a compound, has different properties compared to those of the elements oxygen and hydrogen from which it is formed. Justify this statement.
See AnswerHydrogen is a combustible gas and oxygen supports combustion.
But when they chemically combine in a fixed ratio (2:1), they form water, which extinguishes fire.
Thus, the properties of water are completely different from the properties of hydrogen and oxygen.
This is a characteristic feature of compounds.
4. In which of the following cases are all the examples correctly matched? Give reasons in support of your answers.
(i) Elements — water, nitrogen, iron, air.
(ii) Uniform mixtures — minerals, seawater, bronze, air.
(iii) Pure substances — carbon dioxide, iron, oxygen, sugar.
(iv) Non-uniform mixtures — air, sand, brass, muddy water.
See Answer(i) Incorrect. (Water and air are mixtures/compounds, not elements).
(ii) Incorrect. (Minerals are usually compounds, not uniform mixtures).
(iii) Correct. (Carbon dioxide, iron, oxygen and sugar are all pure substances).
(iv) Incorrect. (Air is a uniform mixture, brass is an alloy = uniform).
So, only (iii) is correctly matched.
5. Iron reacts with moist air to form iron oxide and magnesium burns in oxygen to form magnesium oxide. Classify all the substances involved in the above reactions as elements, compounds or mixtures, with justification.
See AnswerIron → Element
Moist air → Mixture (air + water vapour)
Iron oxide → Compound
Magnesium → Element
Oxygen → Element
Magnesium oxide → Compound
Class 8 Science Curiosity Chapter 8 Question 6 and 7
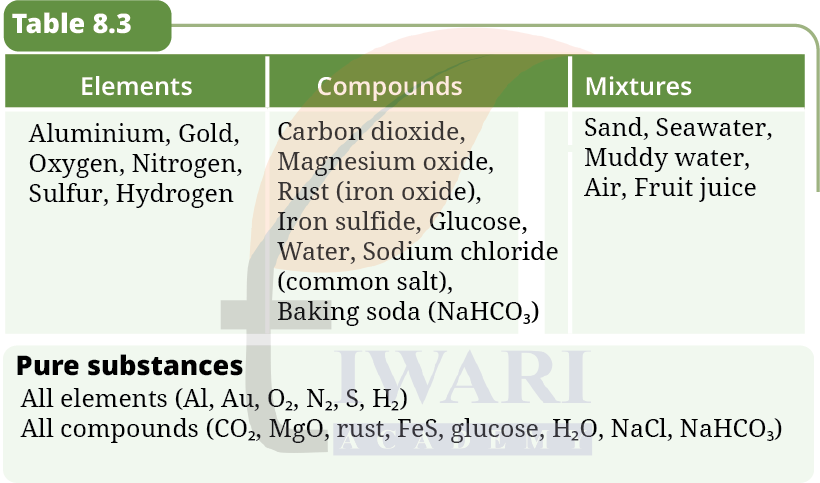
6. Classify the following as elements, compounds, or mixtures in Table 8.3. Carbon dioxide, sand, seawater, magnesium oxide, muddy water, aluminium, gold, oxygen, rust, iron sulfide, glucose, air, water, fruit juice, nitrogen, sodium chloride, sulfur, hydrogen, baking soda
Answer:
7. What new substance is formed when a mixture of iron filings and sulfur powder is heated, and how is it different from the original mixture? Also, write the word equation for the reaction.
See Answer• When heated, iron filings and sulfur combine to form iron sulfide (FeS).
• Difference:
• Original mixture: Components (iron + sulfur) retain properties, can be separated with a magnet.
• Product: Iron sulfide is a compound with completely new properties, cannot be separated.
Word equation:
Iron + Sulfur → Iron sulfide
Question 8, 9 and 10 of Class 8 Science Curiosity Chapter 8
8. Is it possible for a substance to be classified as both an element and a compound? Explain why or why not.
See Answer• No, a substance cannot be both at the same time.
• Element → made of only one kind of atom (e.g., O₂, H₂, Fe).
• Compound → formed by chemical combination of two or more elements (e.g., H₂O, CO₂).
Therefore, classification is mutually exclusive.
9. How would our daily lives be changed if water were not a compound but a mixture of hydrogen and oxygen?
See Answer• If water were just a mixture of hydrogen and oxygen gases, they would not chemically combine.
• The mixture would be explosive and unsafe for drinking or daily use.
• Life would not be possible because water’s unique properties (solvent, temperature regulation, etc.) come from being a compound (H₂O), not a mixture.
10. Analyse Fig. 8.24. Identify Gas A. Also, write the word equation of the chemical reaction.
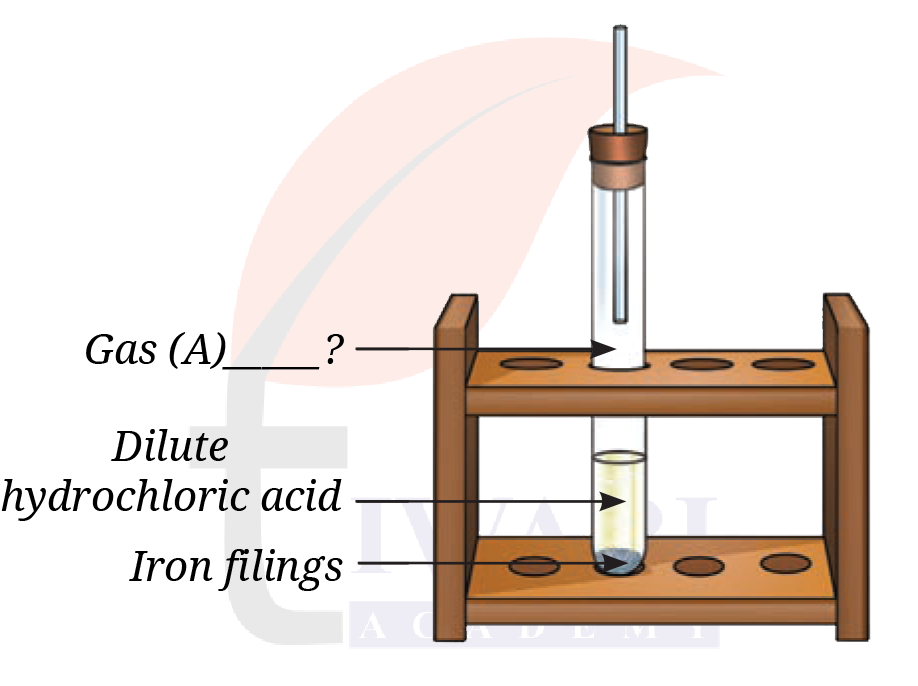
See AnswerFig. 8.24 shows iron filings reacting with dilute hydrochloric acid.
Gas A = Hydrogen gas (H₂).
Word equation:
Iron + Hydrochloric acid → Iron chloride + Hydrogen
Class 8 Science Curiosity Chapter 8 Question 11 and 12
11. Write the names of any two compounds made only from non-metals, and also mention two uses of each of them.
See Answer1. Carbon dioxide (CO₂):
• Used in fire extinguishers.
• Used in making fizzy (aerated) drinks.
2. Sulfur dioxide (SO₂):
• Used in bleaching and as a preservative.
• Used in the manufacture of sulfuric acid.
12. How can gold be classified as both a mineral and a metal?
See Answer• As a mineral: Gold occurs naturally in the earth’s crust in native (pure) form, so it is a mineral.
• As a metal: Gold is also an element with metallic properties like lustre, ductility and conductivity.
Hence, gold is classified as both a mineral and a metal.




