NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom in Hindi and English Medium prepared for new session 2024-25. Class 9 Science chapter 4 is modified and revised as per the new rationalised NCERT textbooks issued for academic year 2024-25.
Class 9 Science Chapter 4 Question Answers
- Class 9 Science Chapter 4 Exercises
- Class 9 Science Chapter 4 Intext Questions
- Class 9 Science Chapter 4 Extra Questions
- Class 9 Science Chapter 4 Hindi Medium
- Class 9 Science Chapter 4 Notes in English
- Class 9 Science Chapter 4 Notes in Hindi
- Class 9 Science Chapter 4 NCERT Book
- Class 9 Science NCERT Solutions
- Class 9 all Subjects NCERT Solutions
| Class: 9 | Science |
| Chapter 4: | Structure of the Atom |
| Contents: | Intext, Exercise and Extra Questions |
| Mode of Content: | Text, Videos and PDF Format |
| Medium: | English and Hindi Medium |
NCERT Class 9 Science textbook, Chapter 4 Structure of the Atom delves into the structure and components of atoms, providing insight into the fundamental building blocks of matter. The main topics covered in Chapter 4 are the concept of the atom as the basic unit of matter, Dalton’s Atomic Theory that is a brief overview of Dalton’s atomic theory and its postulates. Here we will study about Charged Particles in Matter which provides the explanation of charged particles in matter, including electrons, protons, and neutrons.
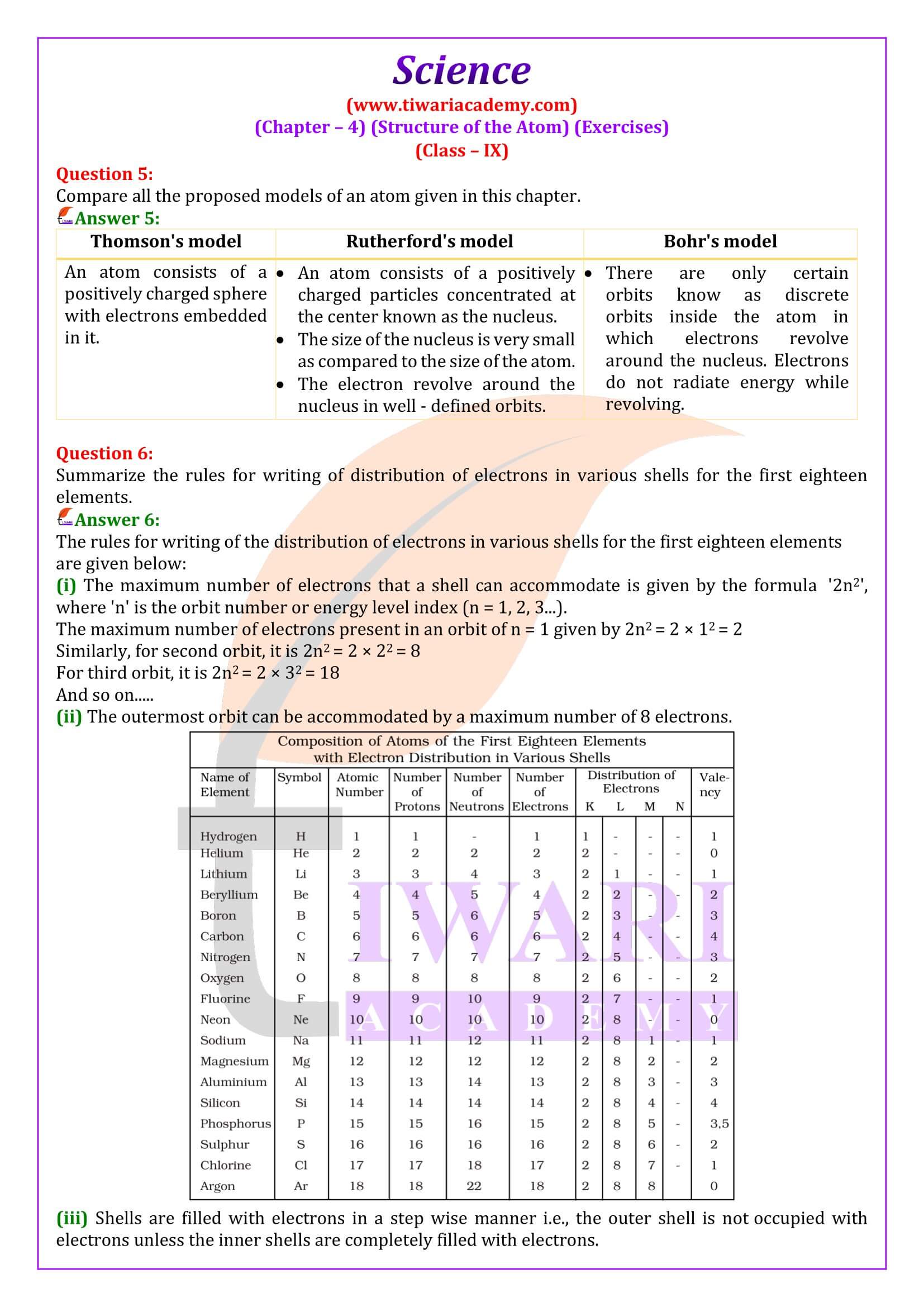
Distribution of Electrons in Different Orbits (Shells)
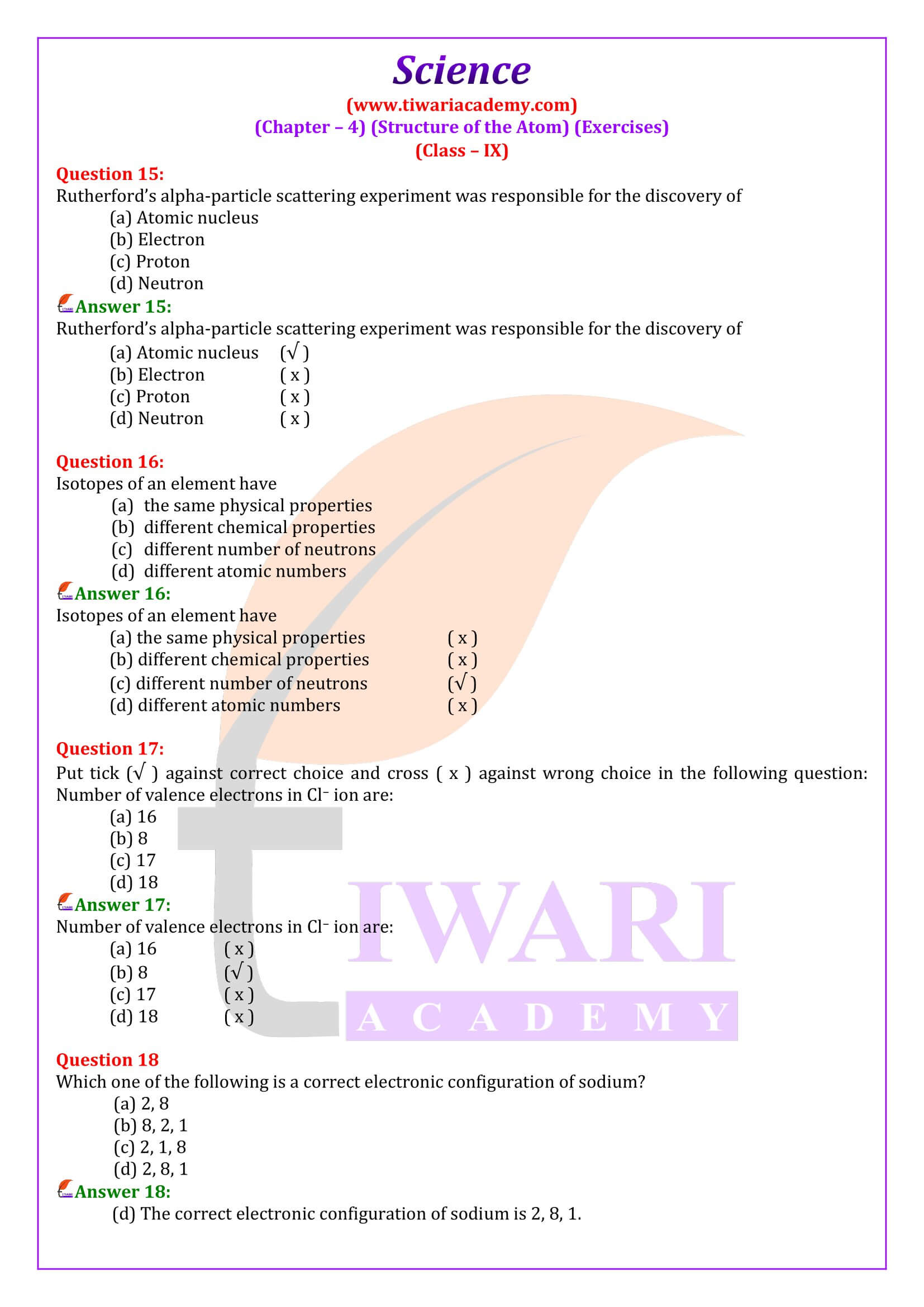
Class 9 students learn in chapter 4 about explanation of electron distribution in shells or energy levels and introduction to the K, L, M, and N shells. It also explains the definition of valency and its significance, determining valency based on electron distribution. Here, we learn about Atomic Number and Mass Number with explanation of atomic number and its significance, definition of mass number and how it is determined. Class 9 science chapter 4 explores about the definition of isotopes and examples, explanation of isotopic notation.
NCERT Solutions for Class 9 Science Chapter 4
A recap of the main concepts and ideas presented in Chapter 4 Structure of the Atom, provides a foundational understanding of the structure and components of atoms. It introduces students to the historical development of atomic models and sets the stage for further exploration of atomic and molecular structures in subsequent chapters and classes.
Preparing for NCERT Class 9 Science Chapter 4 Structure of the Atom, requires a proper approach to understanding the fundamental concepts related to atomic structure. Here are some steps and planning strategies for students to prepare this chapter 4 of 9th science effectively. First of all, read the Chapter Thoroughly, begin by reading the chapter carefully from the NCERT textbook. Pay attention to definitions, examples, and key points. While reading, take detailed notes on important concepts, definitions, and key terms.
To make science easy, summarize the main ideas of each section. Understand the historical development of atomic models, including Dalton’s atomic theory, J.J. Thomson’s experiments, Rutherford’s gold foil experiment, and Bohr’s model. Know the key contributions of these scientists. Study the components of an atom, including electrons, protons, and neutrons. Understand their properties, locations, and charges. Learn about Bohr’s model of the hydrogen atom, including the concept of energy levels or orbits and how it explains spectral lines.
Class IX Science chapter 4 Exercise and Intext question answers in PDF format to free download. Class 9 UP Board students also get here UP Board Solutions. Class 9 Science chapter 4 Intext questions given on Page 43 or Page 49 or Page 50 or Page 52 or Page 53 and Exercises in English Medium are available.
You can download these solutions from Page 53 ke Uttar or Page 56 ke Uttar or Page 57 ke Uttar or Page 58 ke uttar or Page 59 ke Uttar or Page 60 ke Uttar and Abhyaas ke Uttar to study online or in PDF file format. NCERT Solutions and Offline Apps for all other subjects based on latest CBSE Syllabus are also available to download.
Electron Distribution and Valency
Understand how electrons are distributed in different shells or energy levels (K, L, M, N). Learn the maximum number of electrons each shell can hold. Know the definition of valency and how it relates to electron distribution. Practice determining the valency of elements based on their electron configuration. Learn how to calculate the number of protons, electrons, and neutrons in an atom. Isotopes: Understand isotopic notation.
Practice solving numerical problems related to atomic structure, atomic number, mass number, and electron distribution. Work through exercises and examples in the textbook. Utilize diagrams and visual aids in the chapter to help understand atomic structures and models. Supplement your learning with online resources, videos, and interactive simulations related to atomic structure. These can provide additional clarity. Group study can offer different perspectives and help reinforce your understanding.
Regularly revise your notes and the key points of the chapter 4 in 9th science. Repetition is key to retaining information. Closer to your exams, practice solving sample papers and previous year’s question papers to familiarize yourself with the exam pattern and practice time management. If you have doubts or find certain topics challenging, don’t hesitate to seek help from your teacher or classmates. Clarify your doubts as soon as possible. By following these planning strategies and staying organized, you can effectively prepare for NCERT Class 9 Science Chapter 4 and build a strong foundation in atomic structure and chemistry concepts.
Extra Questions on 9th Science Chapter 4
Is atomic number of an atom always equal to the number of electrons?
No, it is the case when the atom has no charge. In case of cation (positively charged), atomic number is more than the number of electrons and in case of anion (negatively charged), it is less than the number of electrons.
Nucleus of an atom has positive charge on it. Establish.
This can be established on the basis of Rutherford scattering experiment. Since some alpha particles were repelled by the nucleus of the atom, it is expected to have the same charge as on alpha particles. Therefore, nucleus of an atom has positive charge.
Were neutrons known at the time Rutherford performed the scattering experiment?
No, these were discovered later on by Chadwick in 1931 whereas scattering experiment was performed by Rutherford in 1911.
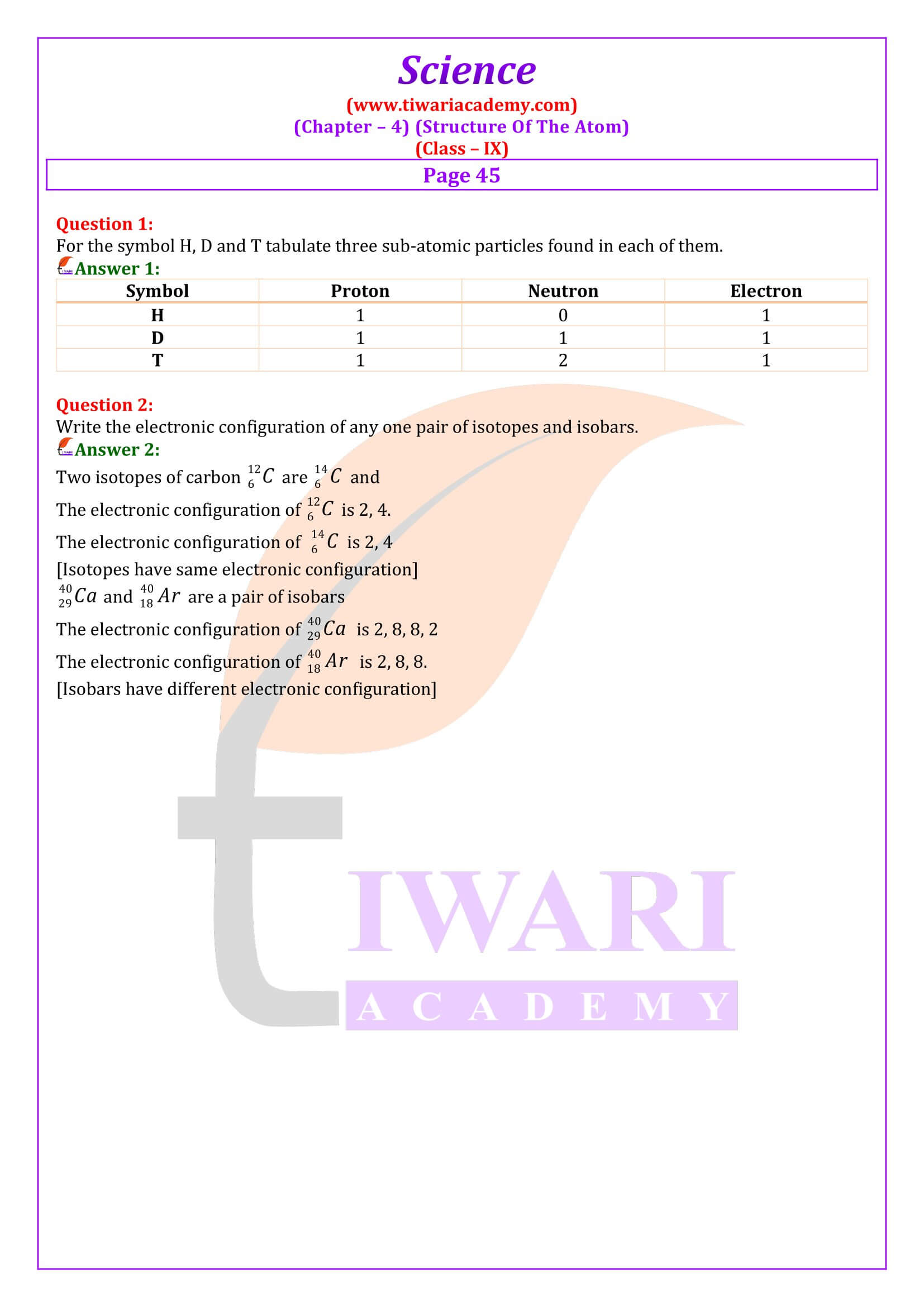
Why are isotopes of an element chemically similar?
Isotopes of an elements have same number of electrons and therefore, same value shell electronic distribution. Since the chemical properties of the atoms are related to valence shell configurations, the isotopes are chemically similar.
Why do elements which exist as isotopes have fractional atomic masses?
The different isotopes of an element differ in their mass number as well as atomic masses. In order to represent the atomic mass of the element, we have to consider average of the atomic masses of the different isotopes and also the ratio in which these are present. In most of the cases, the average comes out to be in fraction. Therefore, these elements have fractional atomic masses.
Why is a proton not a universal particle like electron?
A proton is the positively charged residue left when hydrogen gas is enclosed in the in the discharge tube. For the other gases, the positive residues formed contain different number of protons. Therefore, proton is not a universal particle like electron.
Tiwari Academy plays a significant role in helping students prepare for NCERT Class 9 Science Chapter 4. Here’s how Tiwari Academy can assist students in their preparation for this chapter. It provides detailed solutions to all the questions and exercises in the NCERT Class 9 Science textbook for Chapter 4. These solutions can help students understand how to approach and solve different types of problems related to the structure of atoms.
Important Questions on 9th Science Chapter 4
What are the limitations of J.J. Thomson’s model of the atom?
According to J.J. Thomson’s model of an atom, an atom consists of a positively charged sphere with electrons embedded in it. However, it was later found that the positively charged particles reside at the center of the atom called the nucleus, and the electrons revolve around the nucleus.
What are the limitations of Rutherford’s model of the atom?
According to Rutherford’s model of an atom, electrons revolve around the nucleus in fixed orbits. But, an electron revolving in circular orbits will not be stable because during revolution, it will experience acceleration. Due to acceleration, the electrons will lose energy in the form of radiation and fall into the nucleus. In such a case, the atom would be highly unstable and collapse.
Define valency by taking examples of silicon and oxygen.
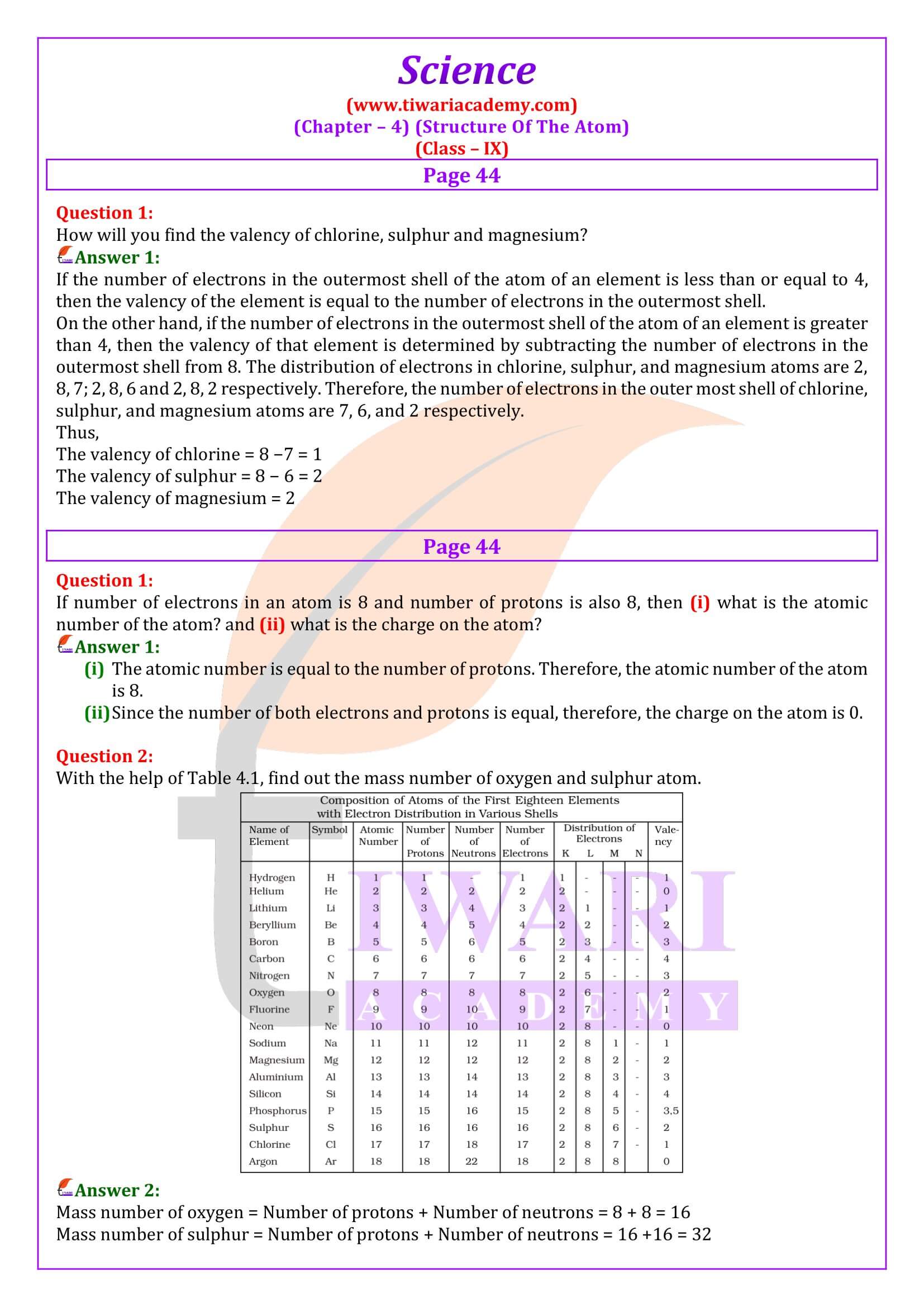
The valency of an element is the combining capacity of that element. The valency of an element is determined by the number of valence electrons present in the atom of that element. If the number of valence electrons of the atom of an element is less than or equal to four, then the valency of that element is equal to the number of valence electrons. For example, the atom of silicon has four valence electrons. Thus, the valency of silicon is four. On the other hand, if the number of valence electrons of the atom of an element is greater than four, then the valency of that element is obtained by subtracting the number of valence electrons from eight. For example, the atom of oxygen has six valence electrons. Thus, the valency of oxygen is (8 − 6) i.e., two.
Explain Atomic number with examples.
Atomic number: The atomic number of an element is the total number of protons present in the atom of that element. For example, nitrogen has 7 protons in its atom. Thus, the atomic number of nitrogen is 7.
What do you understand by isobars?
Isobars: They are atoms of different elements having same mass number but different atomic number. For example calcium, atomic number 20 and argon, atomic number 18. The number of electrons in these atoms is different, but the mass number of both these elements is 40. That is, the total number of neutrons is the same in the atoms of this pair of elements.
If Z = 3, what would be the valency of the element? Also, name the element.
By Z = 3, we mean that the atomic number of the element is 3. Its electronic configuration is 2, 1. Hence, the valency of the element is 1 (since the outermost shell has only one electron). Therefore, the element with Z = 3 is lithium.
9th Science Chapter 4 Answers in English and Hindi Medium
NCERT Solutions for Class 9 Science Chapter 4 Structure of the Atom Intext Questions and chapter end exercise question answers are given below to free download. All the solutions are free to download without any registrations or login. We have updated all the contents on the basis of user’s suggestions and Feedback received from last academic session.
The Tiwari Academy website and apps offer explanations and videos that break down complex concepts in the chapter. Visual aids and clear explanations can help students grasp difficult topics more easily. It also provide historical context and explanations for the development of atomic models, making it easier for students to understand the evolution of atomic theory. The platform offer visual representations and interactive simulations of atomic structures, which can enhance students’ understanding of the arrangement of electrons, protons, and neutrons within an atom.
Questions related to class 9 science chapter 4, atomic structure and atomic models are frequently included in Class 9 Science examinations. Students can expect to see questions about atomic components, electron distribution, atomic number, and mass number in their exams. The chapter covers the historical development of atomic models, including Dalton’s atomic theory, J.J. Thomson’s experiments, Rutherford’s gold foil experiment, and Bohr’s model. Understanding the history of atomic theory is often tested in exams.
Questions for Practice on 9th Science Chapter 4
Question 1:
An electron is regarded as a universal particle. Explain.
Answer 1:
The value of charge (e) and mass (m) of the electron always remain the same whatever may be the source of their emission. In the discharge tube, the electrons may be emitted either from the cathode or form the gas enclosed in the discharge tube. Whatever may be the metal which forms the cathode or the gas present in the discharge tube, these values remain the same. Therefore, electron is regarded as a universal particle.
Question 2:
Why do the element helium, neon and argon have zero valency?
Answer 2:
Helium has two electrons in its only energy shell (K-shell). The other two elements have eight electrons in their valence shells. Since these are the maximum number of electrons which the atoms of these elements can have therefore, they do not have any urge or desire to take part in chemical combination. These elements are known as zero valent elements. They have therefore, valency equal to zero.
Class 9 Science chapter 4 bridges the gap between chemistry and physics by introducing concepts related to atomic structure. It sets the stage for students to explore topics like the periodic table, chemical bonding, and atomic physics in later classes. Knowledge of atomic structure is relevant to various scientific fields, including chemistry, physics, and materials science. Exams include questions that require students to apply their understanding of atomic structure to real-world scenarios. A strong foundation in Chapter 4 is, therefore, essential for success in future science studies.
Importance of Class 9 Science Chapter 4
Finally, the NCERT Class 9 Science Chapter 4, Structure of the Atom, is important not only for scoring well in exams but also for laying the groundwork for a deeper understanding of chemistry and physics concepts. Students should dedicate time and effort to comprehensively learn the concepts presented in this chapter to ensure success in their science studies.