NCERT Textbook Solutions for Class 9 Science Chapter 1 Updated for New Academic Session 2025-26, Matter in Our Surroundings Intext and Exercises question answers. Get here the solutions of Page 3, Page 6, page 9, page 10 and Exercises question answers in English Medium and Hindi Medium.
NCERT Solutions for Class 9 Science Chapter 1
Class 9 Science Chapter 1 Answers in English Medium
- Class 9 Science Chapter 1 Exercises
- Class 9 Science Chapter 1 Intext Questions
- Class 9 Science Chapter 1 MCQ
- Class 9 Science Chapter 1 Extra Questions
- Class 9 Science Chapter 1 Hindi Medium
- Class 9 Science Chapter 1 Notes in English
- Class 9 Science Chapter 1 Notes in Hindi
- Class 9 Science Chapter 1 NCERT Book
- Class 9 Science NCERT Solutions
- Class 9 all Subjects NCERT Solutions
NCERT Class 9 Science textbook, Chapter 1 Matter in Our Surroundings covers the most of the important topics. Definition of Matter Characteristics of Matter, three states of matter – Solid, Liquid and Gas Comparison of the characteristics of these states Interconversion of States of Matter.
The main topics of class 9 science chapter 1 Change of state melting, freezing, evaporation and condensation Effect of change of temperature on the states of matter.
Evaporation: Factors affecting the rate of evaporation Applications of evaporation.
Sublimation: Definition of sublimation Examples of substances that sublime.
Diffusion: Definition of diffusion Examples of diffusion in daily life Characteristics of Particles of Matter.
Download App for Class 9 all Subjects free. UP Board Solutions, NCERT Textbook Solutions, NCERT Exercise Solutions Offline Apps 2025-26 are free to download for all students using latest NCERT Books 2025-26. Here you can use NCERT Solutions of chapter 1 of Class 9 Science online or download in PDF file format for offline use. Download Class 9 Science Solutions Apps in Hindi & English version for offline use.
Particles in solids, liquids, and gases. Kinetic Theory of Matter: Explanation of the kinetic theory Explanation of the behavior of particles in matter according to the kinetic theory.
Measurement of Matter: Mass and weight measuring the mass of an object Units of measurement.
Density and Relative Density: Definition of density Calculation of density Relative density and its applications.
These are the main topics covered in Chapter 1 of the NCERT Class 9 Science textbook. This chapter provides an introduction to the fundamental concepts related to matter and its various properties. It serves as the foundation for further exploration of chemistry and physics topics in the curriculum.
Preparing for NCERT Class 9 Science Chapter 1 Matter in Our Surroundings, or any chapter, requires a systematic approach to understanding the concepts and practicing them effectively. Here are some tips on how students can prepare for this chapter in a better way. Start by reading the chapter carefully from the NCERT textbook. Pay attention to the text, diagrams and examples provided. While reading, make concise notes of important concepts, definitions and key points. This will help you with quick revision.
| Class: 9 | Science |
| Contents: | NCERT Solutions and Important Questions |
| Chapter 1: | Matter in Our Surroundings |
| Content Type: | Text, PDF and Videos |
| Session: | Year 2025-26 |
| Medium: | English and Hindi Medium |
9th Science Chapter 1 Answers in English and Hindi Medium
NCERT Solutions for Class 9 Science Chapter 1 Matter in Our Surroundings all the in-text questions as well as chapter end exercises question’s answers are given below. Solutions are available in Hindi and English Medium to study online or download in PDF form free.
Ensure that you have a clear understanding of fundamental concepts such as matter, states of matter and the kinetic theory of matter. Use diagrams and illustrations provided in the book to visualize how particles are arranged in different states of matter and during phase changes. Work through the examples and exercises provided at the end of the chapter. Practice solving numerical problems related to density and other concepts to reinforce your understanding.
If you find any topics challenging, consult additional resources like reference books, online tutorials, or videos to clarify your doubts. If you have questions or face difficulties in understanding certain concepts, don’t hesitate to seek help from your teacher or discuss them with your classmates. Where possible, perform simple experiments related to states of matter, diffusion or density to gain practical insights into the concepts. Create Flashcards: Create flashcards for important terms, definitions, and formulas. This can help you with quick revision.
Consistent practice is key to mastering any subject. Allocate regular study time to science and review the chapter periodically. Take self-assessment quizzes or practice tests to gauge your understanding of the chapter. A few days before your exams, revise the chapter thoroughly. Focus on your notes and important points. Keep your notes, textbooks and other study materials well-organized so that you can easily access them for review.
Science is about exploring and asking questions. Stay curious and be open to learning new concepts and ideas. Remember that understanding the fundamental concepts in Chapter 1 of Class 9 Science is useful as it forms the basis for more advanced topics in chemistry and physics in the later chapters. Building a strong foundation in this chapter will benefit you throughout your science studies.
Tiwari Academy is an online educational platform that provides resources and materials to help students prepare for their CBSE (Central Board of Secondary Education) Class 9 Science exams, including Chapter 1. Tiwari Academy offers free access to a wide range of study materials, including NCERT solutions, textbooks, revision notes and practice papers. These materials are designed to help students understand and revise the content effectively.
Class 9 Science Chapter 1 Extra Practice Questions
Why do substance undergo change in physical state?
Substance undergo change in physical state because both inter-particle spaces and inter-particle forces can be changed by changing the condition of temperature and pressure.
When sugar is dissolved in water, there is hardly an increase in volume. Which characteristic of matter is illustrated by this observation?
This is because of the presence of inter particle space or empty spaces. Particles or molecules of water can fill the empty space in the particles or molecules of sugar and vice versa. That is why there is hardly any change in the volume as a result of dissolution of sugar in water.
Define gaseous state of a substance.
A substance is said to be in the gaseous state if under normal pressure, its boiling point is below the room temperature.
How does pressure help in the liquefication of a gas?
Increase in pressure helps in the liquefication of a gas. The particles or molecules of a gas come closer and closer as the pressure is being increased gradually. They ultimately condense and as a result, the gas liquefies or changes into the liquid state.
Solids are generally very heavy while gases are light. Explain.
In the solids, the particle are very closely packed. As a result, the number of particles per unit volume is quite large. Therefore, the solids are normally quite heavy. In the gases, the particles are loosely packed. The number of particle per unit volume is completely small. Therefore, gases are light.
Tiwari Academy offers comprehensive NCERT solutions for Class 9 Science Chapter 1, guiding students step-by-step through exercises in the NCERT textbook. These solutions are invaluable for students, helping them decipher the best ways to tackle diverse problems. The platform might also feature video tutorials that break down intricate concepts of the chapter. By using visual tools and explanations, students can more easily understand challenging subjects.
Questions for Practice on 9th Science Chapter 1
By working through additional exercises and questions, students solidify their comprehension and enhance their problem-solving capabilities. Tiwari Academy typically provides succinct and organized revision notes, which are instrumental for quick recaps of the chapter’s main ideas and crucial details. Some digital platforms, including Tiwari Academy, even have online exams that emulate the actual test atmosphere, which can be instrumental for student evaluation and exam readiness.
Question 1:
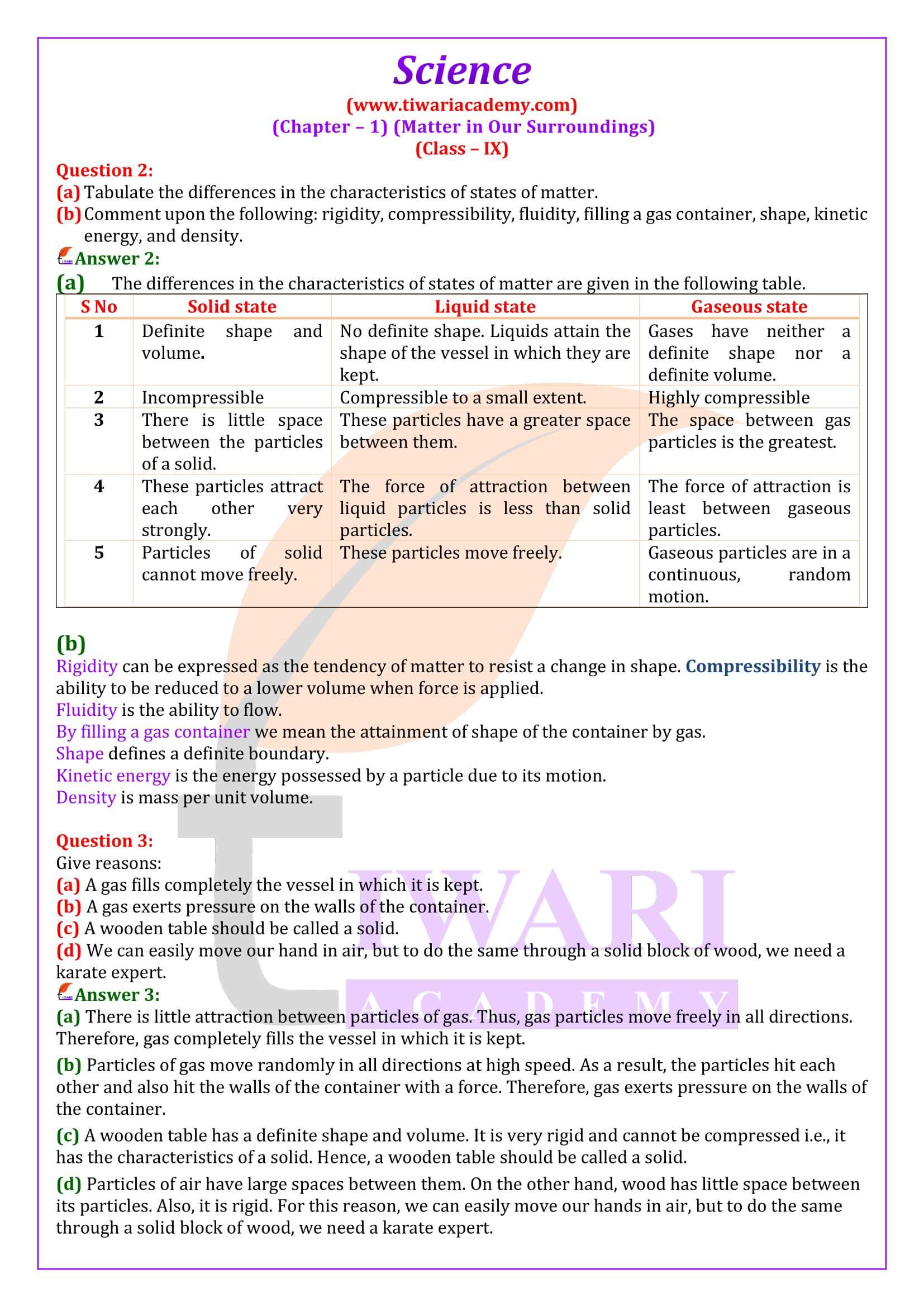
Solids are normally not compressible. Why can a sponge be readily pressed?
Answer 1:
A sponge made up of rubber has a large number of fine pores in which air remains filled. When the sponge is pressed, the air from the pores escapes and vacant space are left. Therefore, the sponge can be readily pressed on applying pressure.
Accessible around the clock, Tiwari Academy’s digital tools let students learn at a rhythm that suits them. This constant availability is especially beneficial for those who wish to revisit the chapter several times. Doubt Resolution: Certain online educational platforms grant students the opportunity to post their chapter-related queries. These queries might be addressed by seasoned educators or tutors, offering students needed explanations. Additionally, Tiwari Academy might have archives of past exam papers with their solutions. Working on these papers can assist students in acclimating to the exam structure and honing their time-management skills.
Question 2:
Why do we sweat on a humid day?
Answer 2:
In humid day, the air around us has already high percentage of water vapours. Therefore, the water coming from the skin gets less opportunity to charge into vapours and remains sticking to our body. We therefore, swear more on a humidity day.
Tiwari Academy online platforms have discussion forum where students can interact, share their experiences, and seek advice from peers. It’s important to note that the availability of specific resources and features may vary on Tiwari Academy or any other educational platform. Students should explore the platform to determine which resources are most helpful for their individual learning needs and preferences. Using a combination of resources, including the official NCERT textbook, school notes and online materials like Tiwari Academy, can provide a comprehensive approach to exam preparation.
From an examination point of view, NCERT Class 9 Science Chapter 1 Matter in Our Surroundings, holds significant importance for several reasons. Chapter 1 introduces fundamental concepts related to matter, states of matter and the kinetic theory of matter. These concepts serve as the foundation for understanding more advanced topics in chemistry and physics in higher classes. A strong grasp of these basics is crucial for building a solid scientific knowledge base.
Question 3:
Kelvin scale of temperature is regarded as better than the Celsius scale. Give reason.
Answer 3:
In the Celsius scale of temperature we often come across a negative sign for the temperature (e.g., -8.5⁰C). Since the sign is always positive in the Kelvin scale, it is regarded as better.
Questions from 9th science chapter 1 are often included in Class 9 Science examinations. This chapter is considered an essential part of the curriculum and students can expect to see questions related to states of matter, properties of matter and related calculations in their exams. Chapter 1 helps students develop a deeper understanding of how matter behaves, how particles are arranged in different states and how temperature affects matter. This conceptual understanding is not only essential for exams but also for a broader understanding of science.
The chapter 1 of 9th science includes numerical problems related to density and the states of matter. These problems require students to apply mathematical concepts to scientific scenarios, which is a valuable skill for both exams and real-world applications. The concepts discussed in Chapter 1 are applicable to various everyday situations, such as cooking, weather changes and material properties. Understanding these applications can help students relate science to their daily lives and answer practical questions in exams.
Important Questions on 9th Science Chapter 1
Naphthalene balls disappear with time without leaving any solid. Why?
Naphthalene shows the property of sublimation. Evaporation of naphthalene takes place easily and so it disappears during course of time without leaving a solid.
We can get the smell of perfume sitting several meters away. Why?
Perfumes vaporize very fast and its vapours diffuse into air easily. That is why we can smell perfume sitting several meters away.
Water at room temperature is a liquid. Give reason.
Water at room temperature is a liquid because it has fluidity and has definite volume but no definite shape.
An iron almirah is a solid at room temperature. Give reason.
An iron almirah is a solid at room temperature because it is rigid and has a definite shape.
Why is ice at 273 K more effective in cooling than water at the same temperature?
Ice at 273 K is less energetic than water. It is because of the difference in the latent heat of fusion which is present in water at the same temperature in the form of extra energy.
What produces more severe burns, boiling water or steam?
Steam produces more severe burns than boiling water. This is because steam has more energy than boiling water, present in it in the form of latent heat of vaporization.
Successfully mastering the first chapter of the textbook can boost a student’s confidence in their ability to handle the rest of the science curriculum. It sets a positive tone for the subject and encourages students to explore further. The knowledge gained from Chapter 1 will be built upon in higher classes. Topics like states of matter, kinetic theory and properties of matter are revisited and expanded upon in later chapters and classes.
A strong foundation in Chapter 1 is, therefore, essential for future success. In summary, NCERT Class 9 Science Chapter 1 is not only important for scoring well in exams but also for laying the groundwork for a deeper understanding of science in the later stages of education. Students should dedicate time and effort to comprehensively learn the concepts presented in this chapter to ensure a strong foundation in science.