Class 10 Science Chapter 2 Solutions
Class 10 Science Chapter 2 Exercises Solutions
Class 10 Science Chapter 2 Intext Exercises
Class 10 Science Chapter 2 in Hindi Medium
Class 10 Science Book Download in PDF
Class 10 Science Chapter 2 Notes
Class 10 Science Chapter 2 Board Questions
Class 10 Science Chapter 2 MCQ
Class 10 Science Chapter 2 Extra Questions
Class 10 Science NCERT Solutions
Class 10 all Subjects Solutions
NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases and Salts in Hindi and English Medium updated for CBSE session 2024-25. In NCERT Class 10 Science, Chapter 2 Acids, Bases, and Salts, chapter covers various topics related to acids, bases, and salts, which are fundamental concepts in chemistry.
Here is an outline of the chapter 2 covered in NCERT Class 10 Science Chapter 2. Definition and basic properties of acids and bases. Get here the revised solutions of chapter 2 class 10th science based on new syllabus and textbooks issued for academic year 2024-25.
| Class: 10 | Science |
| Chapter 2: | Acids, Bases and Salts |
| Content: | Exercise Solutions and Extra Questions |
| Content Type: | Online Text and Videos Format |
| Session: | CBSE 2024-25 |
| Medium: | Hindi and English Medium |
Class 10 Science Chapter 2 Answers in Hindi and English Medium
- Class 10 Science Chapter 2 Exercises
- Class 10 Science Chapter 2 Intext Questions
- Class 10 Science Chapter 2 in Hindi
- Class 10 Science Chapter 2 in PDF
- Class 10 Science Chapter 2 Notes
- Class 10 Science Chapter 2 Board Questions
- Class 10 Science Chapter 2 MCQ
- Class 10 Science Chapter 2 Extra Questions
- Class 10 Science NCERT Solutions
- Class 10 all Subjects Solutions
Chemical Properties of Acids and Bases
Explanation of various chemical properties of acids (e.g., corrosiveness, reaction with metals, and litmus paper test). Chemical properties of bases (e.g., reaction with metals, reaction with acids, and litmus paper test). Use of indicators to test for acidity and basicity (e.g., litmus paper, pH paper, and universal indicator). Importance of pH scale in measuring acidity and basicity.
Strength of Acids, Bases and Salt
Differentiating between strong and weak acids and bases. Explanation of concentration and ionization in the context of strong and weak acids/bases. The importance of common salt (sodium chloride, NaCl) in daily life. Production of various chemicals from common salt (e.g., sodium hydroxide, chlorine, and baking soda).

Uses of Acids and Bases and Preparation of Salts
Various industrial and domestic applications of acids and bases. The role of acids and bases in food preservation and digestion. Methods for preparing salts, including Neutralization reactions (e.g., acid + base → salt + water). Solubility rules for predicting the formation of precipitates. Understanding the pH scale in detail. pH values of common substances and their Classification as acidic, neutral, or basic. It’s important to refer to the NCERT textbook you are using for any updates or additional details related to the chapter.
| Class 10 All Subjects iOS App for iPad & iPhone |
Importance of pH and Neutralization
How pH plays a crucial role in various processes and products, such as agriculture, health, and industry. Concept of neutralization reactions. Balanced chemical equations for neutralization reactions. Learn here how the concentration of hydrogen ions (H⁺) is related to the pH of a solution. Importance of Self-Defense in Animals and Plants that How some animals and plants use acids and bases for self-defense. These are the main topics typically covered in NCERT Class 10 Science Chapter 2 Acids, Bases, and Salts.
NCERT Solutions for Class 10 Science Chapter 2
Preparing for NCERT Class 10 Science Chapter 2, Acids, Bases, and Salts, or any chapter in a more effective manner involves a structured and thorough approach. Here are some steps and tips to help students prepare for this chapter: Start by reading the chapter from your NCERT textbook to grasp the fundamental concepts. Pay attention to definitions, properties, and examples of acids, bases, and salts.
Just like English Medium, Hindi Medium solutions of chapter 2 intext questions Page 20 ke Uttar or Page 24 ke Uttar or Page 27 ke Uttar or Page 31 ke Uttar or Page 36 ke Uttar or Abhyas ke Prashn Uttar are given to use free updated for new academic session 2024-25. Download CBSE Solutions Apps in Hindi & English Medium. These NCERT Solutions are based on latest CBSE Curriculum. 10th Science chapter 2 intext questions answers given on Page 18, Page 22, Page 25, Page 28, Page 33 and chapter end Exercises in English Medium and Hindi Medium free to use. Get here Important Questions which are important for Board Exams, Revision Notes and MCQ of Chapter 2 in 10th Science.
Take Notes and Use Visual Aids
While reading, take concise notes summarizing key points, definitions, and equations. Organize your notes for easy reference. Utilize diagrams and charts provided in the textbook to understand the concepts visually. You can also create your own diagrams or flowcharts to visualize complex processes. Solve the exercises and practice problems provided at the end of the chapter.
Focus on Chemical Equations and Use Online Resources
Pay special attention to chemical reactions involving acids, bases, and salts. Practice balancing chemical equations related to neutralization reactions and salt formation. Explore online resources, such as video tutorials and interactive simulations, to reinforce your understanding of acid-base concepts. Allocate regular study time for this chapter to ensure you cover all the topics in a structured manner. Consistency is key to effective learning.
Class 10 Science Chapter 2 Practice Tests with Answers
Understand pH Scale and Use Mnemonics
Thoroughly understand the pH scale and its significance in measuring acidity and basicity. Practice calculating pH values. Perform Experiments: If possible, conduct simple experiments related to acids, bases, and salts to observe reactions firsthand. This practical experience can enhance your understanding. Create mnemonics or memory aids to remember key concepts, formulas, and terms. Mnemonics can be a fun and effective way to recall information during exams.
Discuss with Peers and Practice Previous Year Papers
Discussing concepts with classmates or forming study groups can help you clarify doubts and reinforce your knowledge through discussions. Don’t hesitate to ask your teacher for clarification if you have doubts or questions about any topic within the chapter. Solving previous year’s question papers can give you an idea of the types of questions that might be asked in the exam and help you improve your time management.
Focus on Self-Assessment and Revision
Periodically assess your understanding of the chapter by taking self-assessment quizzes or mock tests. Identify areas where you need further practice. As your exams approach, revise the chapter thoroughly. Focus on your notes, important formulas, and frequently asked questions. By following a structured study plan and using a variety of learning resources, you can prepare for NCERT Class 10 Science Chapter 2 in a better way and perform well in your exams.
Tiwari Academy is an educational platform that provides resources and support for students preparing for various academic exams, including NCERT Class 10 Science Chapter 2, which covers the topic of CBSE Syllabus. Tiwari Academy offers detailed and well-explained NCERT solutions for Class 10 Science Chapter 2. These solutions can help students understand the concepts, solve problems, and prepare for exams effectively.
Tiwari Academy provides video lectures or tutorials covering the topics in Chapter 2. Video lectures can be a helpful visual aid for students to grasp complex concepts and understand practical experiments and demonstrations related to acids, bases, and salts. We often offers a wide range of practice questions and exercises related to Class 10 Science Chapter 2. These questions can help students test their knowledge and improve their problem-solving skills in the context of acids, bases, and salts.
Sample Papers and Previous Year Question Papers
We provide sample papers and previous year’s question papers for Class 10 Science. Practicing these papers can help students get a feel for the exam pattern and become familiar with the types of questions asked in the board exams. We provide concise and well-organized notes and study material related to Chapter 2. Being an educational platform, we offer online tests and quizzes to assess students’ knowledge and track their progress. These tests can help students identify their strengths and weaknesses in the chapter.

Tiwari Academy have a mechanism for students to ask questions and clarify doubts related to Chapter 2. This can be especially helpful for students who are struggling with certain concepts or problems. We offer interactive learning features such as forums, Discussion Forum, chat support where students can interact with teachers and peers, fostering a collaborative learning environment.
From an examination point of view, NCERT Class 10 Science Chapter 2, Acids, Bases, and Salts, holds significant importance. Here’s why this chapter is important for students preparing for Class 10 Science examinations. Questions related to acids, bases, and salts often carry a significant weightage in the Class 10 Science board examinations. This means that a substantial portion of the exam paper is dedicated to this chapter.
10th Science Chapter 2 Answers in English & Hindi Medium
Class 10 Science chapter 2 introduces students to fundamental concepts in chemistry, such as the properties of acids and bases, the pH scale, and chemical reactions involving acids and bases. Mastery of these concepts is crucial for building a strong foundation in chemistry. The knowledge gained from this chapter is not just theoretical; it has practical applications in daily life. Questions related to the uses of acids, bases, and salts in various industries and processes are common in board exams.
NCERT Solutions for Class 10 Science Chapter 2 Acids, Bases and Salts Intext questions and chapter end exercises question answers are given below updated for new academic session 2024-25. NCERT Solutions are given in English and Hindi Medium free to use. Offline Apps 2024-25 are also in updated format free to download. Download Class 10 all Subjects App for mobile use.
Class 10 Science Chapter 2 Extra Question Answers
Define indicators. Name two natural indicators obtained from plants.
Indicators are substances which give different colour in acid or bases solutions. Natural indicators from plants are:
(a) Litmus,
(b) Vanilla extract.
What are antacids?
Antacids are mild alkalies. These are used for getting relief from acidity and indigestion and sometimes, even headache. When taken orally, it reacts with hydrochloric acid present in the stomach and reduces its strength by consuming some of it. For example, milk of magnesia is an antacid.
What are olfactory indicators? Give an example.
Olfactory indicators are substances which have different odour in acid and base solutions. For example, vanilla essence has characteristics pleasant smell in acid solution and no smell in alkali solution.
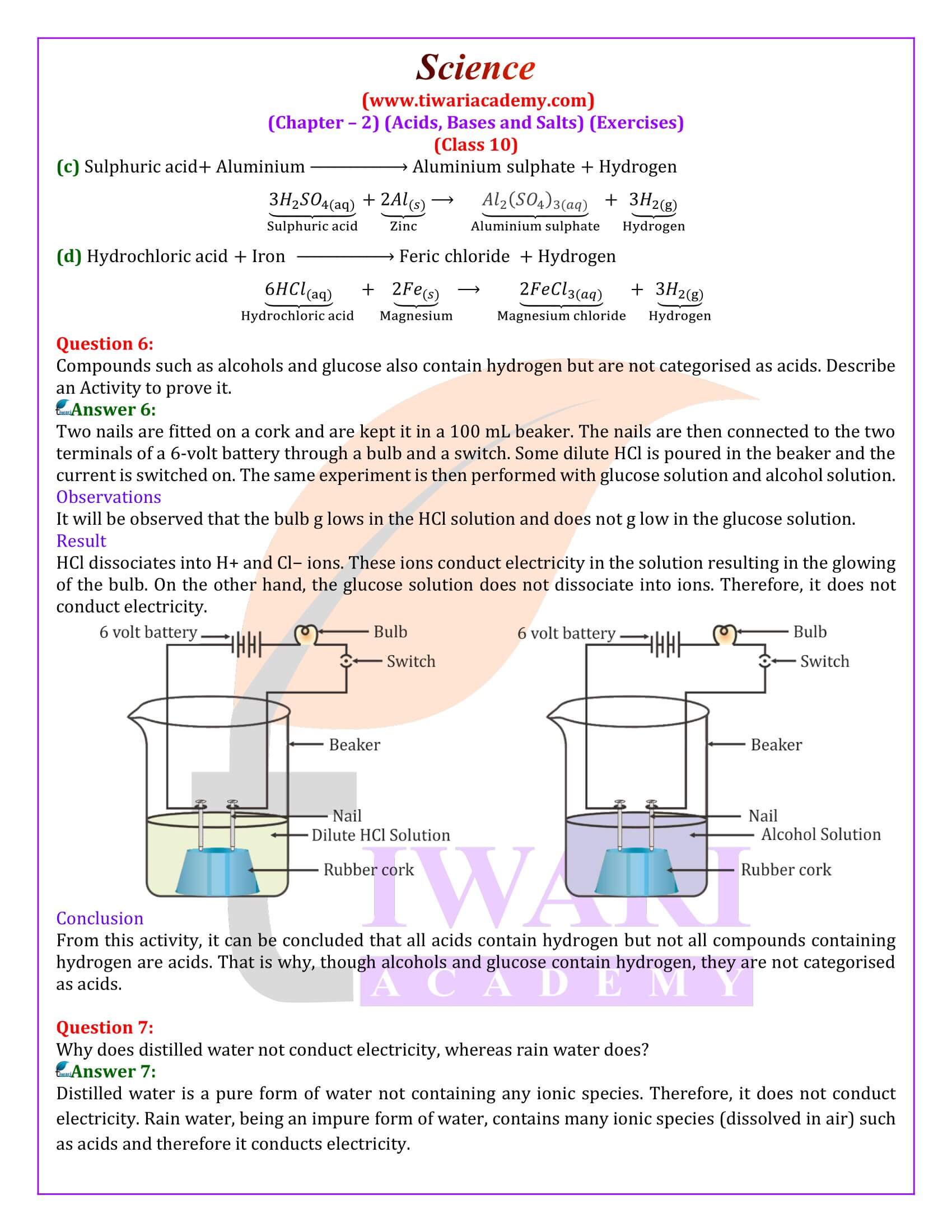
Tap water conducts electricity whereas distilled water does not. Why?
Tap water contains some impurities in the forms of salts. Due to presence of salts, it conducts electricity. Distilled water is free from all kinds of salts and hence does not conduct electricity.
While diluting an acid, why is it recommended that the acid should be added to water and not water into the acid?
When an acid is mixed with water, there is evolution of large amount of heat. Therefore, acid is slowly added to water. If on the other hand, water is added to acid, it might spill on your body and clothes due to explosion and evolution of sudden and large amount of heat.
What is meant by the term pH of a solution? The pH of rain water collected from two cities A and B was found to be 6 and 5 respectively. The water of which city is more acidic?
pH is a term which indicates whether a solution is acidic of basic and to what extent. Mathematically it is a measure of H+ ions concentration in water.
The rain water collected from city B is more acidic.
Tooth enamel is one of the hardest substance in our body. How does it undergo damage due to eating chocolates and sweets? What should we do to prevent it?
Sugar present in chocolates and sweets gets broken to acids by bacteria present in the mouth. This lowers the pH in the mouth. Tooth enamel is made up of calcium phosphate, which gets corroded when the pH in the mouth is below 5.5. To prevent tooth enamel from decay, toothpaste is used because it is alkaline and neutralises the acid produced in the mouth and helps to prevent lowering of pH in mouth.
Why are commercial samples of bleaching powder not completely soluble in water?
Bleaching powder is soluble in water. However, commercial samples of bleaching powder contains slaked lime that does not react with chlorine gas during the manufacture of bleaching powder. The insoluble part of bleaching powder is this white solid, i.e., slaked lime.
We provide additional resources such as animations, diagrams, and illustrations to enhance the understanding of complex topics in 10th Science Chapter 2, particularly those related to chemical reactions and pH. Tiwari Academy offers tips and strategies for effective time management during exam preparation, helping students balance their study schedule with other activities. It’s important for students to explore these resources and choose the ones that align with their learning style and needs. Additionally, students should complement these online resources with their school textbooks and regular study routines to achieve the best results in their Class 10 Science board exams.
Problem Solving and Balanced Chemical Equations
Students are often required to solve numerical problems related to acid-base reactions, pH calculations, and concentration in the exam. These problems assess their problem-solving skills and application of concepts. Understanding and balancing chemical equations for reactions involving acids, bases, and salts is a key part of this chapter. Questions related to chemical equations are commonly asked.
Questions for Practice on 10th Science Chpater 2
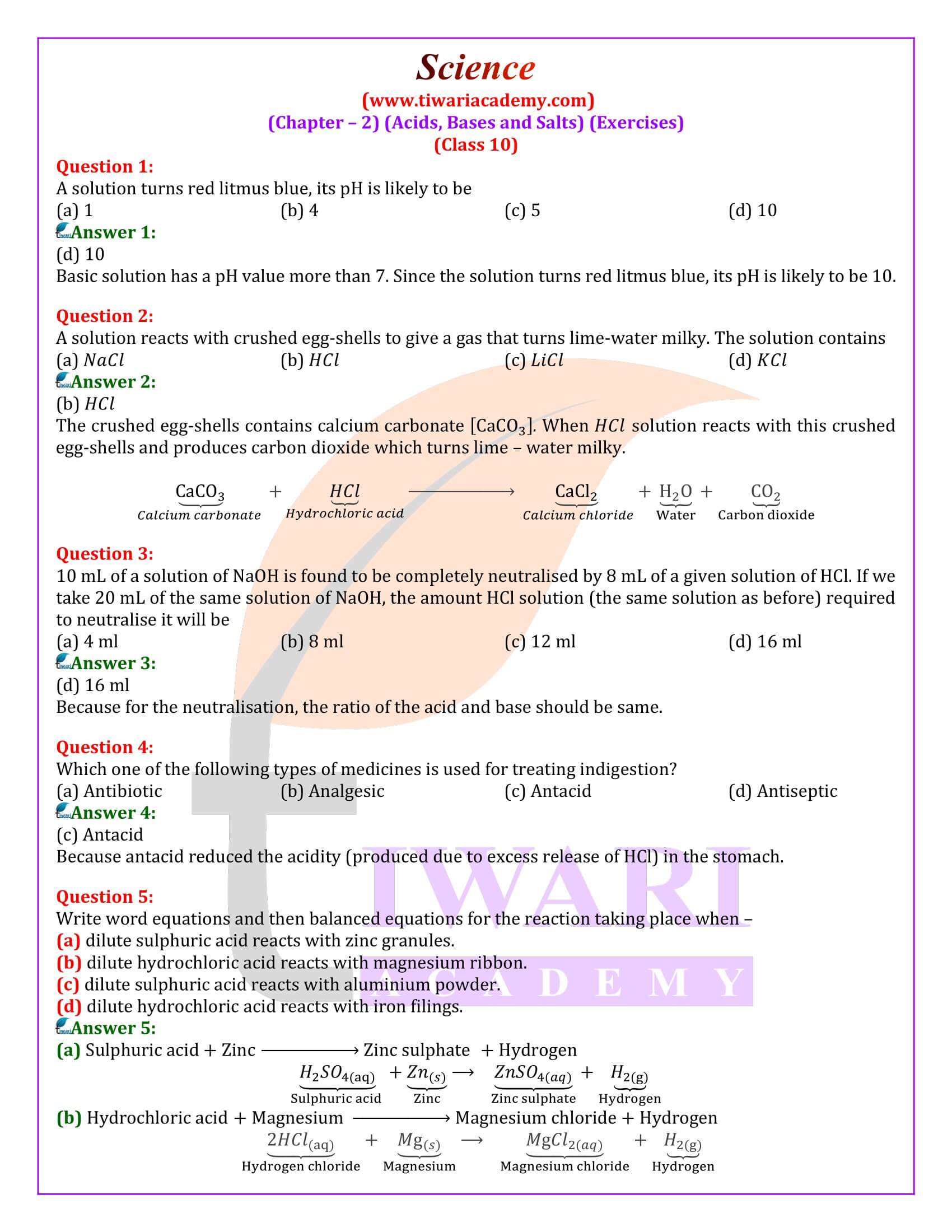
Question 1:
What is a neutralization reaction? Give some examples.
Answer 1:
When the effect of a base is nullified by an acid and vice versa, it is called neutralization reaction. In general, a neutralization reaction is written as:
Base + Acid ―> Salt + Water
Examples:
(a) Aqueous solution of base, NaOH is neutralized by aqueous hydrochloric acid.
(b) Aqueous solution of sulphuric acid is neutralized by aqueous solution of sodium hydroxide.
Some board exams may include questions about experiments related to acids and bases. Students may be asked to describe experimental setups, observations, and inferences. Questions related to pH calculations and the interpretation of pH values are a recurring theme in the exams. Students should be able to determine whether a solution is acidic, neutral, or basic based on its pH. This chapter encourages students to develop practical skills by conducting experiments related to acid-base reactions. Practical skills are often tested in the board exams.
Board exams may include application-based questions that require students to apply their knowledge of acids, bases, and salts to solve real-life problems or scenarios. Understanding the chemical reactions involved in neutralization and salt formation is important for answering questions about the preparation and properties of salts. Beyond rote memorization, this chapter emphasizes conceptual understanding. Students should be able to explain the properties and behavior of acids, bases, and salts.
Question 2:
An alkali is an important base used for the laboratory work. Name the base and state how it can be prepared from common salt? What is this process called?
Answer 2:
An important alkali commonly needed for laboratory work is sodium hydroxide. It can be prepared from sodium chloride by the process of electrolysis. This is called chlor-alkali process.
Electrolysis of aqueous solution of sodium chloride: When electricity is passed through an aqueous solution of sodium chloride commonly called brine, it decomposes into chloride and sodium. Sodium is collected at the cathode where it reacts with water to form sodium hydroxide. Chlorine is formed at the anode and is collected as a gas.
Cathode:
At Anode:
The overall reaction is
Questions from Board Papers
Question 1:
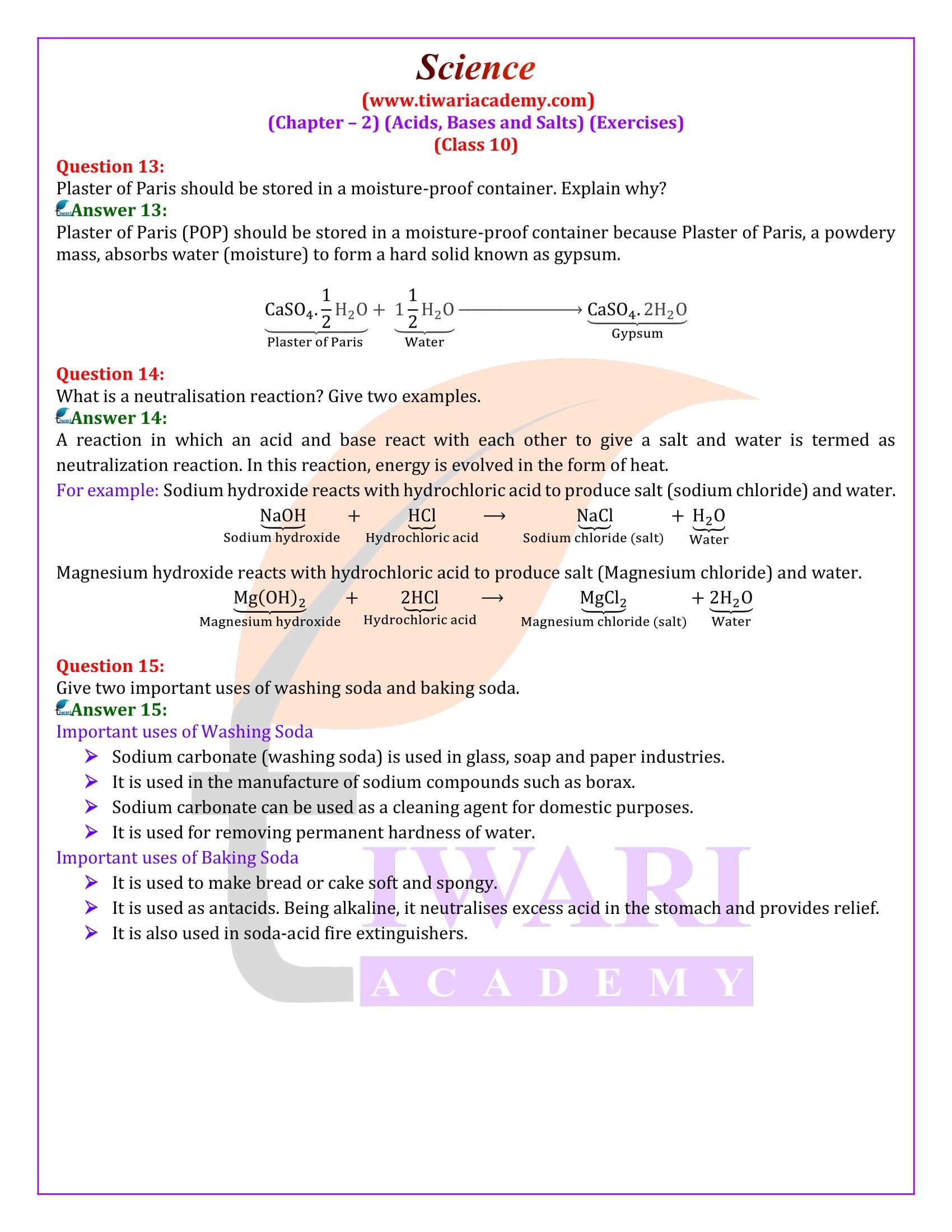
(a) How does baking soda helps to make cakes and bread soft and spongy? OR
Give reason: cake rise on adding baking powder.
(b) Write chemical equation for its preparation.
Answer 1:
(a) On heating , sodium bicarbonate decomposes to produce carbon dioxide. This causes biscuits and cakes etc. to expand and become light. The other constituents act as preservatives.
(b)
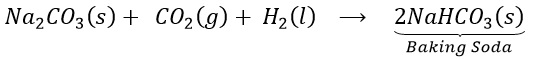
Question 2:
Why does bleaching powder smell strongly of chlorine?
Answer 2:
Bleaching powder smells strongly of chlorine because it slowly reacts with carbon dioxide of air to evolve chlorine gas.
Important Questions on 10th Science Chapter 2
What is a neutralization reaction? Give some examples.
When the effect of a base is nullified by an acid and vice versa, it is called neutralization reaction. In general, a neutralization reaction is written as: Base + Acid ―> Salt + Water
Why does distilled water not conduct electricity, whereas rain water does?
Distilled water is a pure form of water not containing any ionic species. Therefore, it does not conduct electricity. Rain water, being an impure form of water, contains many ionic species (dissolved in air) such as acids and therefore it conducts electricity.
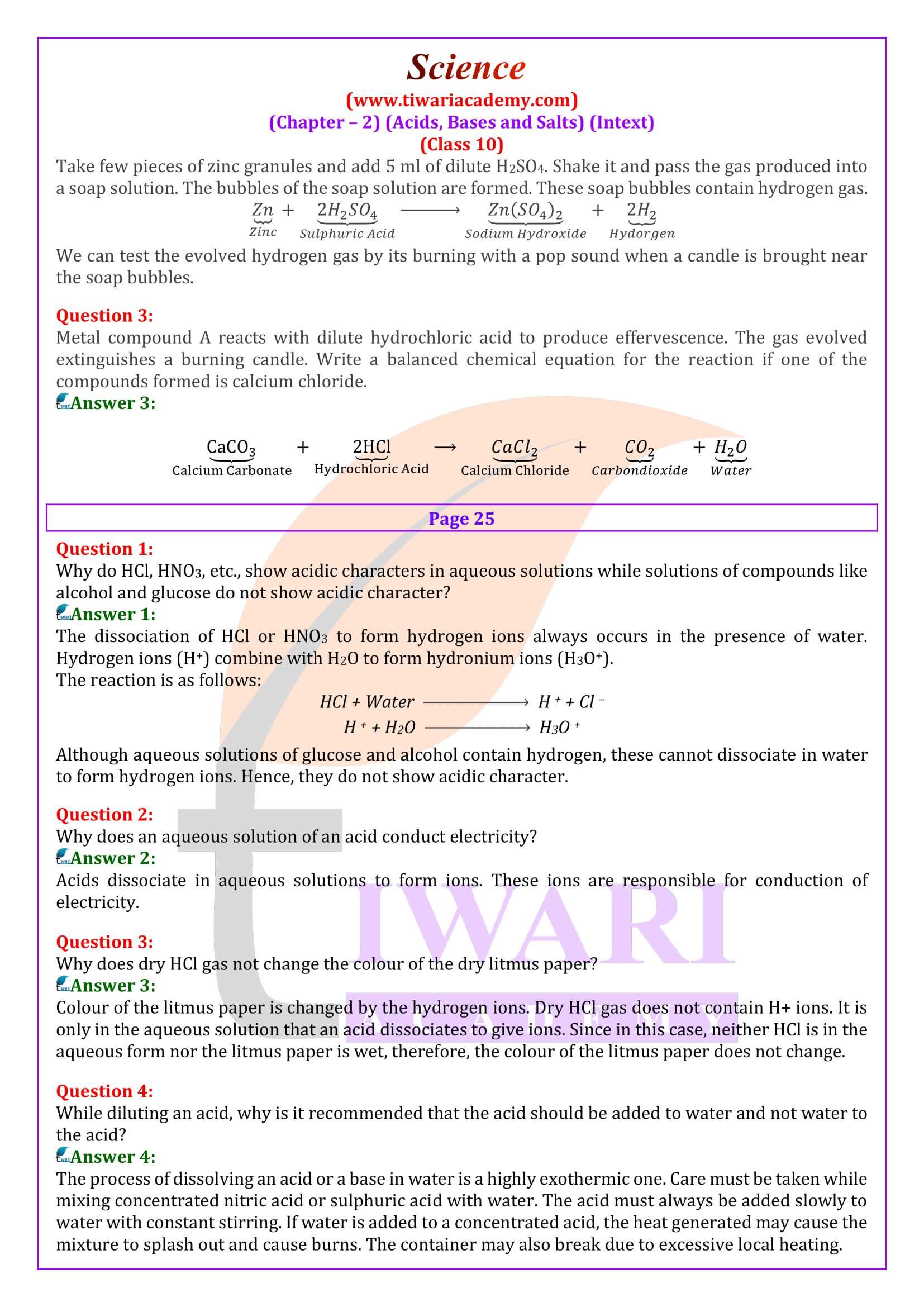
Why do acids not show acidic behaviour in the absence of water?
Acids do not show acidic behaviour in the absence of water because the dissociation of hydrogen ions (H+ ) from an acid occurs in the presence of water only. It is the hydrogen ions (H+ ) that are responsible for the acidic behaviour.
Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain your answer.
The pH of milk is 6. As it changes to curd, the pH will reduce because curd is acidic in nature. The acids present in it decrease the pH.
Why does an aqueous solution of an acid conduct electricity?
Acids dissociate in aqueous solutions to form ions. These ions are responsible for conduction of electricity.
Why does dry HCl gas not change the colour of the dry litmus paper?
Colour of the litmus paper is changed by the hydrogen ions. Dry HCl gas does not contain H+ ions. It is only in the aqueous solution that an acid dissociates to give ions. Since in this case, neither HCl is in the aqueous form nor the litmus paper is wet, therefore, the colour of the litmus paper does not change.
In summary, NCERT Class 10 Science Chapter 2, Acids, Bases, and Salts, is important for board exams because it covers foundational concepts in chemistry and offers practical applications. Students should focus on understanding the principles, mastering problem-solving skills, and being able to apply their knowledge to various scenarios to excel in the examination.
Is chapter 2 of grade 10th Science important from the exam point of view?
Yes, chapter 2 (Acids, Bases, and Salts) of grade 10th Science is important from the exam point of view. Every year questions come from chapter 2 in the exams. There are 34 questions in chapter 2. All the questions of this chapter are significant and can come in the exams. But the most important questions of this chapter that have more chance to come in the board exam are question 3 (page number 22), questions 1, 4 (page number 25), question 1 (page number 28), questions 3, 4 (page number 33), and from back exercise questions 2, 3, 5, 8, 10, 12, 15 are important.
What are the main topics to cover in chapter 2 of class 10th Science NCERT?
After completing chapter 2 of class 10th Science, students will gain the knowledge of the following topics:
- 1. Understanding the chemical properties of acids and bases
a. Acids and Bases in the Laboratory
b. How do Acids and Bases React with Metals?
c. How do Metal Carbonates and Metal Hydrogencarbonates React with Acids?
d. How do Acids and Bases React with each other?
e. Reaction of Metallic Oxides with Acids
f. The reaction of a Non-metallic Oxide with Base - 2. What do all acids and all bases have in common?
a. What Happens to an Acid or a Base in a Water Solution? - 3. How strong are acid or base solutions?
a. Importance of pH in Everyday Life - 4. More about salts
a. Family of Salts
b. pH of Salts
c. Chemicals from Common Salt
d. Are the Crystals of Salts really Dry?
What are the common acids in Chapter 2 of NCERT Class 10 Science that we use in our daily life?
Some acids that we use in our daily life are:
1. Vinegar
2. Orange
3. Tamarind
4. Tomato
5. Curd
6. Lemon
7. Plums
8. Pineapples
9. Coca-cola
10. Apple juice
Is there any activity for CBSE Board Exam in chapter 2 of 10th Science?
There are 15 activities in chapter 2 (Acids, Bases, and Salts) of grade 10th Science. All the activities are nice, interesting, and logical. These activities help students to understand the chapter easily and practically. Students enjoy doing these activities in school.
What is the concept of acid rain in chapter 2 of grade 10th Science textbook?
When the pH of rainwater is less than 5.6, it is known as acid rain. Acid rains are dangerous. The survival of aquatic life becomes difficult.
Is chapter 2 Acids, Bases and Salts of grade 10th Science easy to learn?
Chapter 2 Acids, Bases and Salts of grade 10th Science is not at all challenging. This chapter is overall a simple chapter. However, the difficulty level of any chapter 2 depends on students also. Some students find chapter 2 easy, and some students find chapter 2 difficult.