NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations provides detailed explanations, step-by-step solutions to the exercises and questions provided in the textbook, and additional practice questions to help students understand and master the concepts covered in this chapter.
How to Prepare for Class 10 Science
Class 10 Science Chapter 1 Solutions
Class 10 Science Chapter 1 Exercises Solutions
Class 10 Science Chapter 1 Intext Exercises
Class 10 Science Chapter 1 MCQ
Class 10 Science Chapter 1 in Hindi Medium
Class 10 Science Book Download in PDF
Class 10 Science Chapter 1 Notes
Class 10 Science Chapter 1 Board Questions
Class 10 Science Chapter 1 Extra Questions
Class 10 Science NCERT Solutions
Class 10 all Subjects Solutions
NCERT Solution for Class 10 Science Chapter 1 in English and Hindi Medium for CBSE Session 2025-26 is given here. Get here the updated solutions of chapter 1 class 10 science based on revised syllabus and latest textbooks issued for academic year 2025-26.
| Class: 10 | Science |
| Chapter 1: | Chemical Reactions and Equations |
| Content: | NCERT Exercises and Extra Questions |
| Content Type: | Text and Videos Format |
| Session: | CBSE 2025-26 |
| Medium: | Hindi and English Medium |
Class 10 Science Chapter 1 Solutions in Hindi and English Medium
- Class 10 Science Chapter 1 Exercises
- Class 10 Science Chapter 1 Intext Quesitons
- Class 10 Science Chapter 1 in Hindi
- Class 10 Science Chapter 1 in PDF
- Class 10 Science Chapter 1 Notes
- Class 10 Science Chapter 1 MCQ
- Class 10 Science Chapter 1 Board Questions
- Class 10 Science Chapter 1 Extra Questions
- Class 10 Science NCERT Solutions
- Class 10 all Subjects Solutions
NCERT (National Council of Educational Research and Training) Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations, chapter is part of the NCERT Science textbook for Class 10, which is commonly used in schools following the CBSE (Central Board of Secondary Education) curriculum in Bharat.
These solutions are a valuable resource for students to prepare for their Class 10 Science examinations and build a strong foundation in chemistry. They are typically available in the form of textbooks, online resources, or study guides provided by NCERT or various educational platforms. In this chapter, students learn about chemical reactions and equations, including topics like chemical reactions and their characteristics. Chemical equations and their balancing. Corrosion as a chemical reaction.
Class 10 Science varies by curriculum and education board, but in many common syllabus, Chapter 1 of the Science textbook often covers the following topics:
Chemical Reactions and Equations
Introduction to chemical reactions. Characteristics of chemical reactions (e.g., change in color, formation of a gas, change in temperature). Chemical equations and their significance. Balancing chemical equations. Types of chemical reactions (e.g., combination, decomposition, displacement, double displacement).
Chemical Equations and Balanced Chemical Equations
Explanation of chemical equations and their components. Methods to balance chemical equations. Law of conservation of mass.
Types of Chemical Reactions
Explanation and examples of different types of chemical reactions, including: Combination reactions. Decomposition reactions. Displacement reactions (single and double displacement).
Chemical Reactions and Chemical Equations in Everyday Life
Examples of chemical reactions in everyday life. Balancing chemical equations for common reactions. Chemical reactions involved in the preparation of various substances.
NCERT Solutions for Class 10 Science Chapter 1
Effects of Chemical Reactions
Discussion of the effects and applications of chemical reactions in daily life and industry. Use of chemical reactions in cooking, digestion, and respiration. Download NCERT Solutions as well as NCERT Solutions Apps 2025-26 based on latest NCERT Books which works without internet.
Oxidation and Reduction
Introduction to oxidation and reduction. Redox reactions and their importance. Examples of redox reactions.
Corrosion
Explanation of corrosion. Factors affecting the rate of corrosion. Methods to prevent corrosion. These are the topics covered in NCERT Class 10 Science Chapter 1.
Class 10 Science Chapter 1 Practice Tests with Answers
- 10 Science Chapter 1 Test 1
- 10 Science Chapter 1 Test 1 Answers
- 10 Science Chapter 1 Test 2
- 10 Science Chapter 1 Test 2 Answers
- 10 Science Chapter 1 Test 3
- 10 Science Chapter 1 Test 3 Answers
- 10 Science Chapter 1 Test 4
- 10 Science Chapter 1 Test 4 Answers
- 10 Science Chapter 1 Test 5
- 10 Science Chapter 1 Test 5 Answers
Here are some tips on how a student can prepare Class 10 Science Chapter 1 (Chemical Reactions and Equations) in the best way. Read the NCERT textbook carefully and understand the concepts. Chapter 1 is a relatively short chapter, but it is important to have a good understanding of the basic concepts, such as what a chemical reaction is, how to identify chemical reactions, and how to write and balance chemical equations.
Solve the in-text questions and exercises in the NCERT textbook. This will help you to test your understanding of the concepts and to practice writing and balancing chemical equations.
Use other resources, such as online tutorials, videos, and practice questions. There are many resources available online that can help you to learn the concepts and to practice for your exams.
Discuss the concepts with your classmates or teacher. This is a great way to clarify any doubts and to learn from others. Here are some additional tips.
Pay attention to the key terms and definitions. It is important to be able to define and explain the key terms in the chapter, such as chemical reaction, reactant, product, physical change, chemical change, and element.
Understand the different types of chemical reactions. There are five main types of chemical reactions: combination, decomposition, single replacement, double replacement, and combustion. Be able to identify each type of reaction and to write balanced chemical equations for each type.
Learn how to balance chemical equations. There are many different methods for balancing equations, so find one that works best for you and practice it.
10th Science Chapter 1 Answers in English & Hindi Medium
Apply the concepts you have learned to solve problems. Once you have a good understanding of the concepts, try to apply them to solve problems. You can find practice problems in your textbook, online, and in other resources. By following these tips, students can prepare well for NCERT Class 10 Science Chapter 1 and score good marks in their exams.
NCERT Solutions for Class 10 Science Chapter 1 Chemical Reactions and Equations Exercises and intext questions in English and Hindi Medium updated for new academic session to use online or download in PDF free. All the solutions are updated for UP Board, CBSE Board, MP Board, etc., who are use NCERT Books as course books and following the latest CBSE Curriculum. Download Class 10 all Subjects Solution App free.
Tiwari Academy is an educational platform that provides resources and support for students preparing for various academic exams, including NCERT (National Council of Educational Research and Training) 10th Science Chapter 1. Here’s how Tiwari Academy can help students in their preparation. Tiwari Academy offers detailed and well-explained NCERT solutions for Class 10 Science Chapter 1 and other chapters. These solutions can help students understand the concepts thoroughly and solve questions from the NCERT textbook.
Video Lectures: They provide video lectures and tutorials covering the topics in 10th Science Chapter 1. Video lectures can be a helpful visual aid for students to grasp complex concepts and understand practical experiments and demonstrations.
Tiwari Academy often offers a wide range of practice questions and exercises related to Class 10 Science Chapter 1. These questions can help students test their knowledge and improve their problem-solving skills.
Sample Papers and Previous Year Question Papers: They provide sample papers and previous year’s question papers for Class 10 Science. Practicing these papers can help students get a feel for the exam pattern and become familiar with the types of questions asked in the board exams.
Notes and Study Material: Tiwari Academy provide concise and well-organized notes and study material related to Chapter 1 of 10th Science. These notes can be valuable for quick revision and reference.
Tiwari Academy have a mechanism for students to ask questions and clarify doubts related to Chapter 1 of 10th Science. This can be especially helpful for students who are struggling with certain concepts.
Interactive Learning: Tiwari Academy platform offers interactive learning features such as forums, discussion boards, or chat support where students can interact with teachers and peers, fostering a collaborative learning environment.
Additional Resources: They provide additional resources such as animations, diagrams, and illustrations to enhance the understanding of complex topics in 10th Science Chapter 1.
It’s important for students to explore these resources and choose the ones that align with their learning style and needs. Additionally, students should complement these online resources with their school textbooks and regular study routines to achieve the best results in their Class 10 Science exam.
From an examination point of view, NCERT Class 10 Science Chapter 1, which typically covers the topic of Chemical Reactions and Equations, holds significant importance. Here’s why this chapter is important for students preparing for Class 10 Science examinations.
Foundation of Chemistry
Chapter 1 serves as the foundational chapter for the chemistry portion of the Class 10 Science syllabus. It introduces students to the fundamental concepts of chemical reactions, equations, and types of reactions.
Weightage in Exams
Questions from Chapter 1 are likely to appear in the board examinations. Understanding the content of this chapter is essential to answer questions related to chemical reactions, balancing equations, and types of reactions correctly.
Conceptual Understanding
This chapter lays the groundwork for understanding more advanced topics in chemistry in higher classes. Concepts like balancing chemical equations and identifying types of chemical reactions are essential for building a strong foundation in chemistry.
Application-Based Questions
The concepts covered in 10th Science Chapter 1 are often used to create application-based questions in the board exams. Students may be asked to apply their knowledge of chemical reactions to real-life scenarios.
Numerical Problems
The chapter includes numerical problems related to balancing chemical equations. These problems help students develop problem-solving skills and are commonly included in the exams.
Redox Reactions
Class 10 Science Chapter 1 also introduces the concept of redox reactions (oxidation and reduction), which is a fundamental concept in chemistry. Understanding redox reactions is crucial for higher-level chemistry studies.
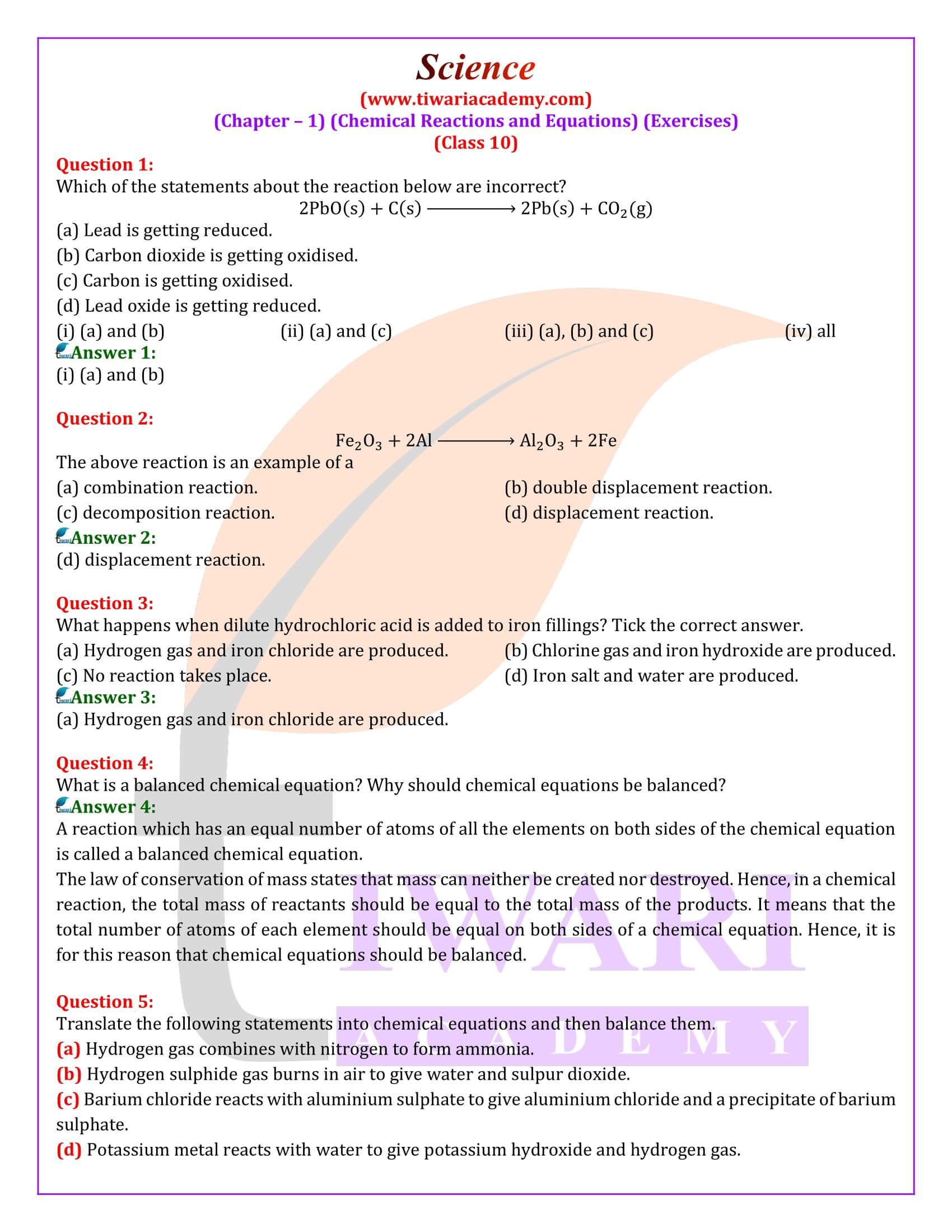
Question 1:
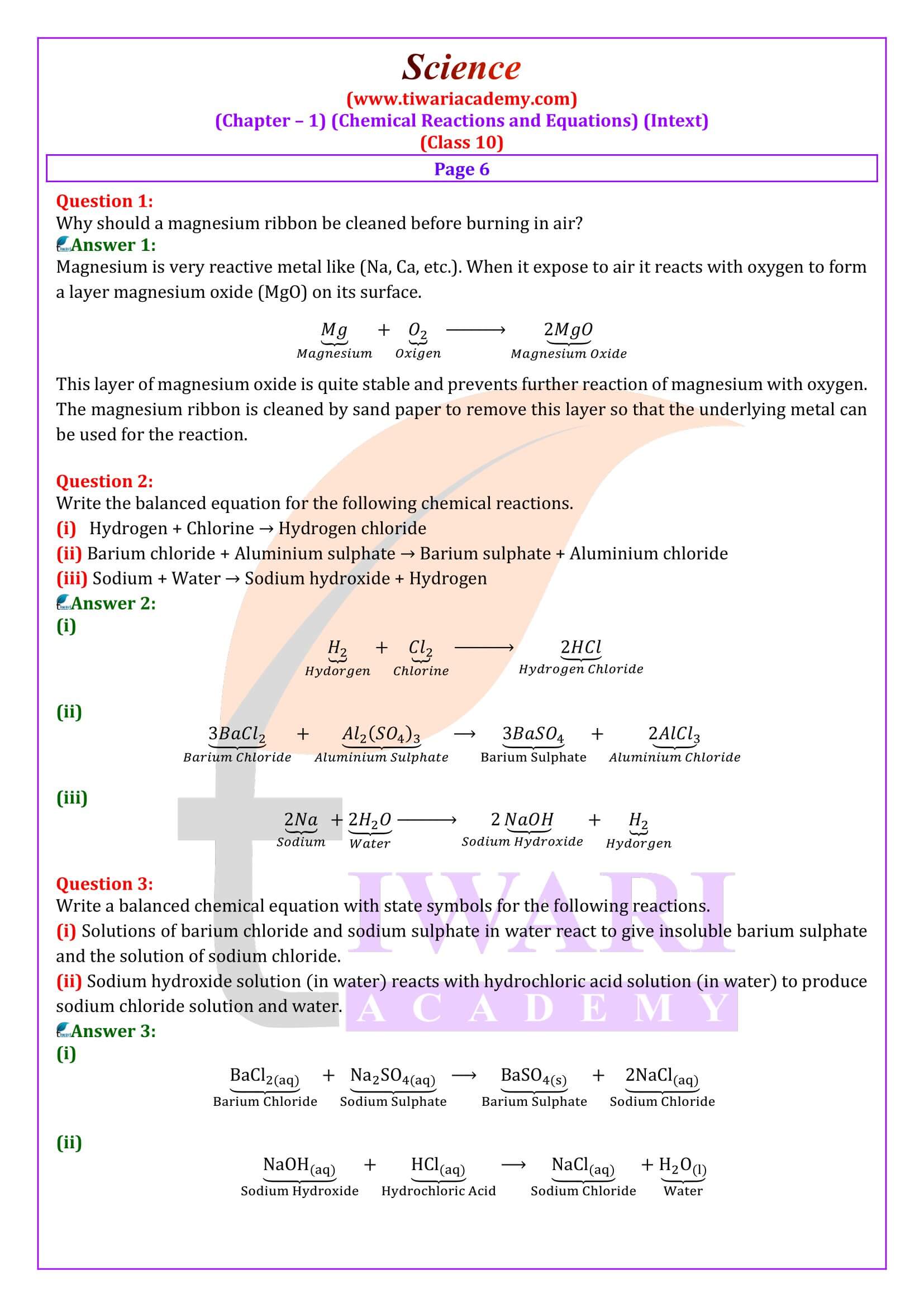
What is meant by a skeletal chemical equation? Using suitable chemical reaction, differentiate between a skeletal chemical equation and a balanced chemical equation.
Answer 1:
If the number of atoms of any element in a chemical equation is not equal on both sides, then it is a skeletal equation. For example:
Here, the number of chlorine and hydrogen atoms are not equal on both sides.
In a Balance equation, the numbers of atoms of different elements on both sides of a chemical equation are equal. For example:
Important Questions on 10th Science Chapter 1
What is a balanced chemical equation? Why should chemical equations be balanced?
A reaction which has an equal number of atoms of all the elements on both sides of the chemical equation is called a balanced chemical equation. The law of conservation of mass states that mass can neither be created nor destroyed. Hence, in a chemical reaction, the total mass of reactants should be equal to the total mass of the products. It means that the total number of atoms of each element should be equal on both sides of a chemical equation. Hence, it is for this reason that chemical equations should be balanced.
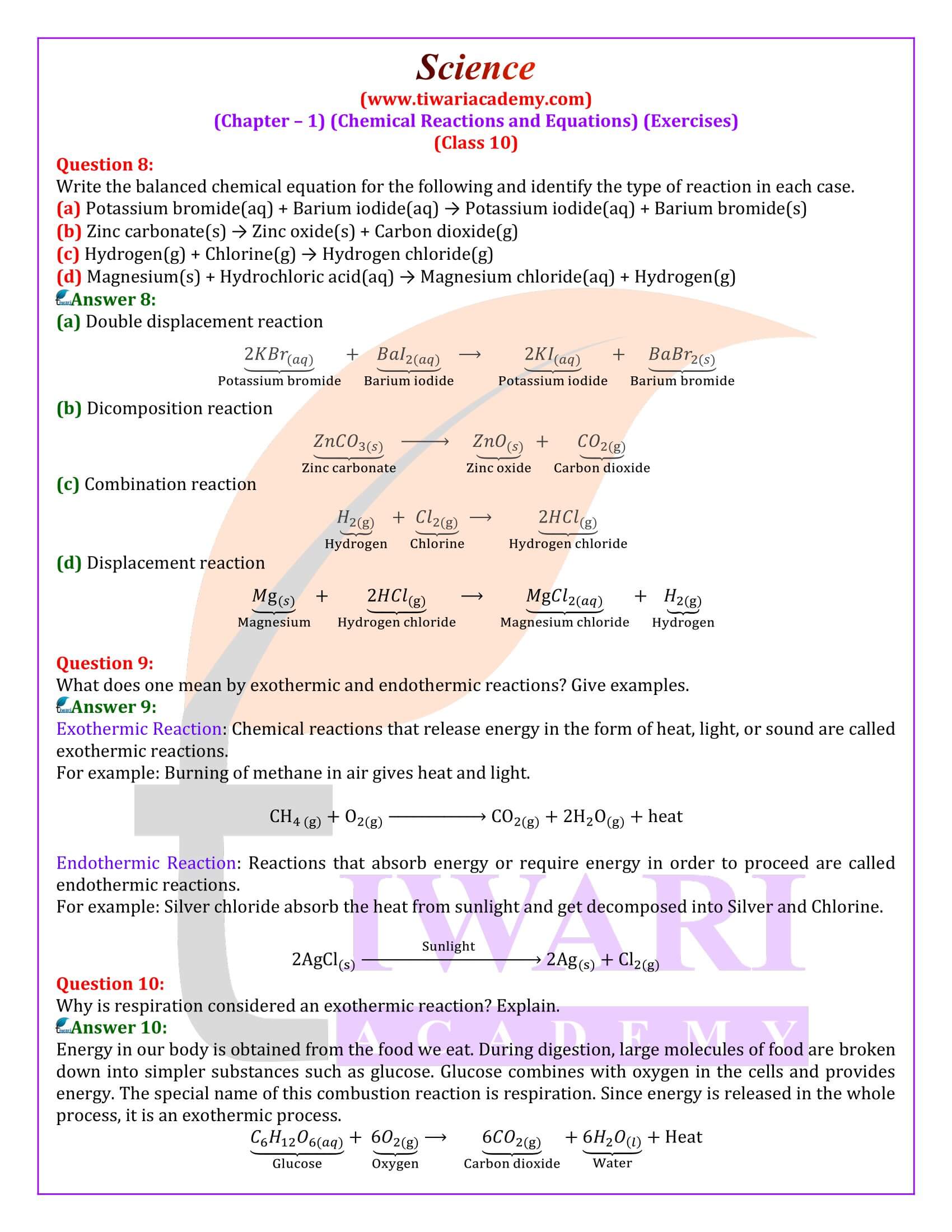
Why is respiration considered an exothermic reaction? Explain.
Energy in our body is obtained from the food we eat. During digestion, large molecules of food are broken down into simpler substances such as glucose. Glucose combines with oxygen in the cells and provides energy. The special name of this combustion reaction is respiration. Since energy is released in the whole process, it is an exothermic process.
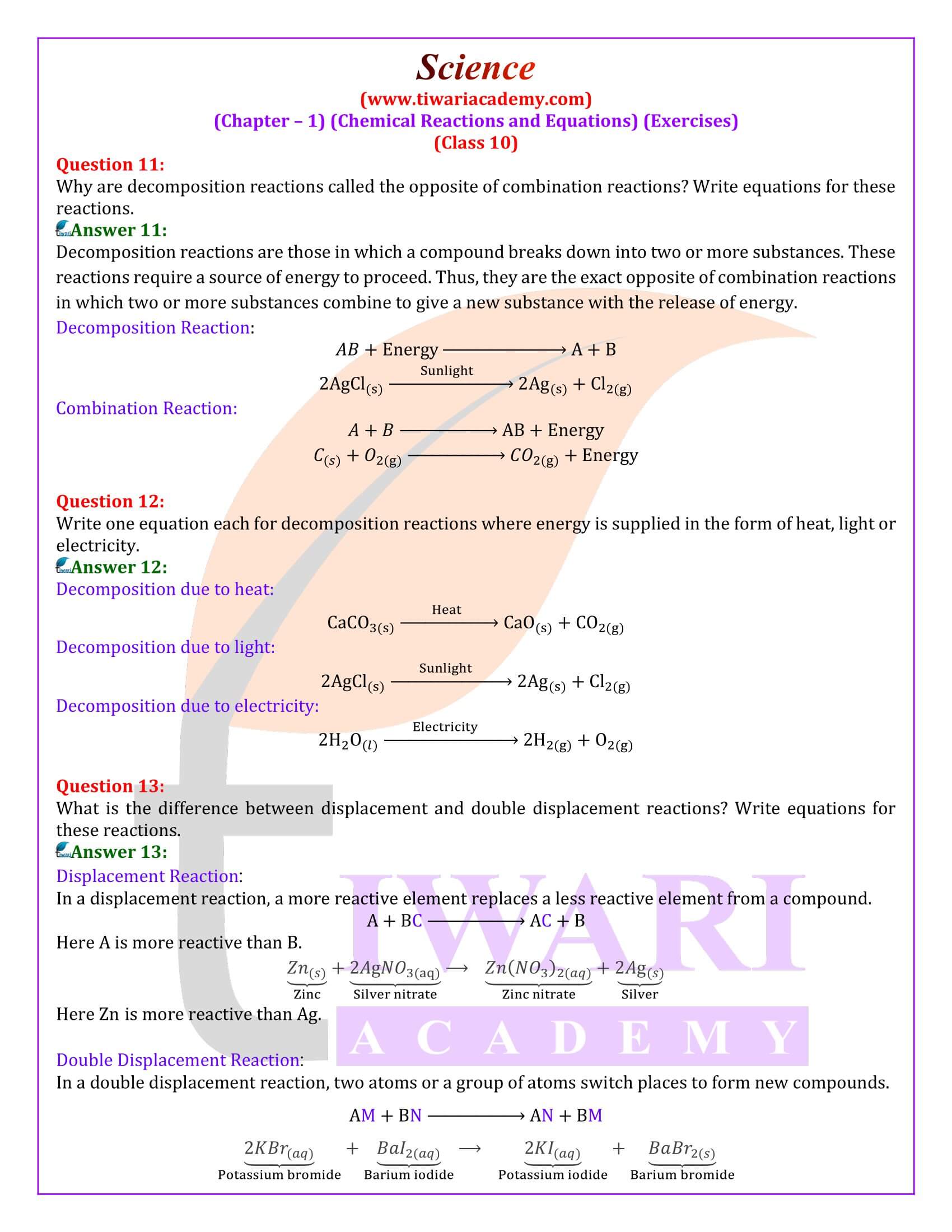
Why are decomposition reactions called the opposite of combination reactions?
Decomposition reactions are those in which a compound breaks down into two or more substances. These reactions require a source of energy to proceed. Thus, they are the exact opposite of combination reactions in which two or more substances combine to give a new substance with the release of energy.
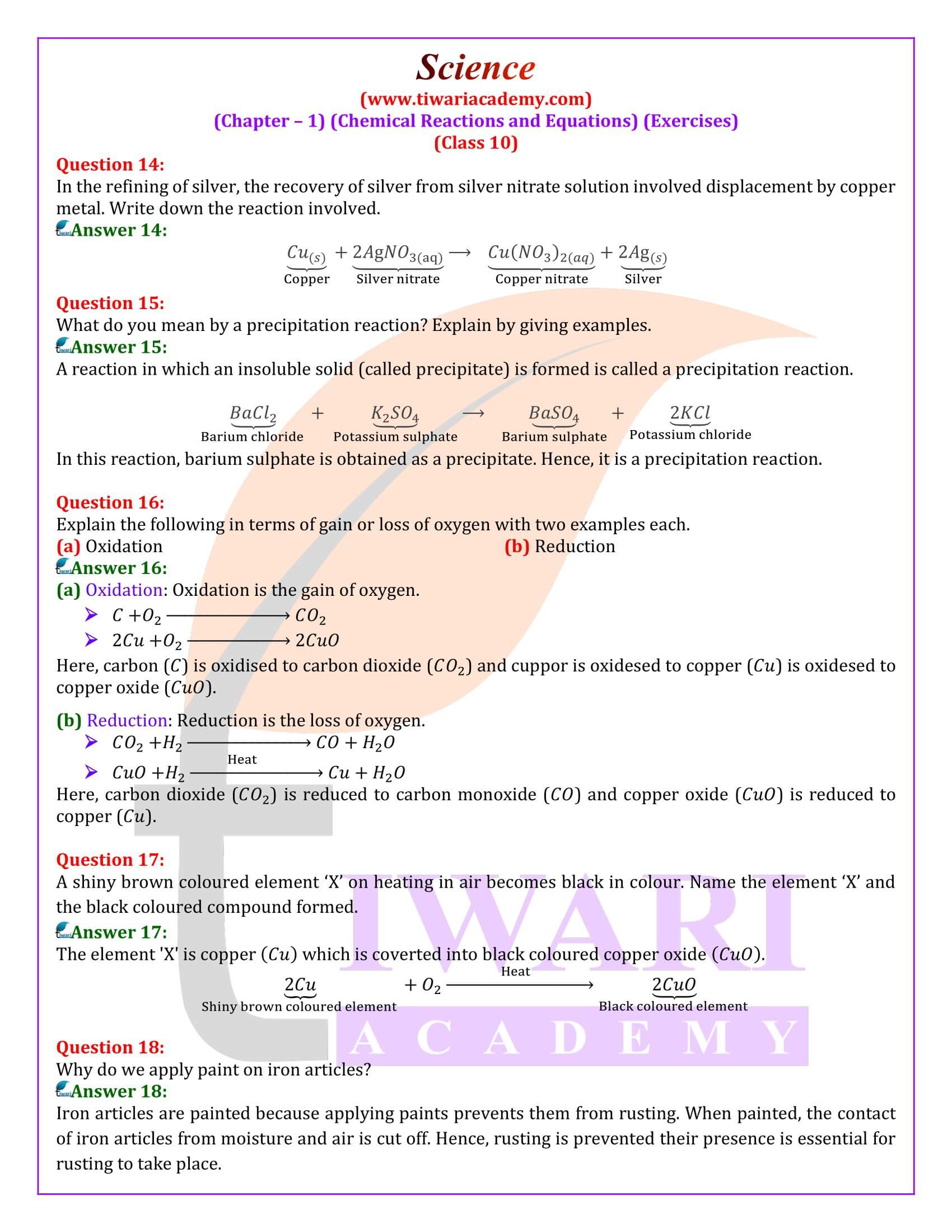
Why do we apply paint on iron articles?
Iron articles are painted because applying paints prevents them from rusting. When painted, the contact of iron articles from moisture and air is cut off. Hence, rusting is prevented their presence is essential for rusting to take place.
Oil and fat containing food items are flushed with nitrogen. Why?
Nitrogen is an inert gas (at room temperature) and does not easily react with oil and fat containing items. On the other hand, oxygen reacts with food substances and makes them rancid. Thus, bags used in packing food items are flushed with nitrogen gas to remove oxygen inside the pack. When oxygen is not present inside the pack, rancidity of oil and fat containing food items is avoided.
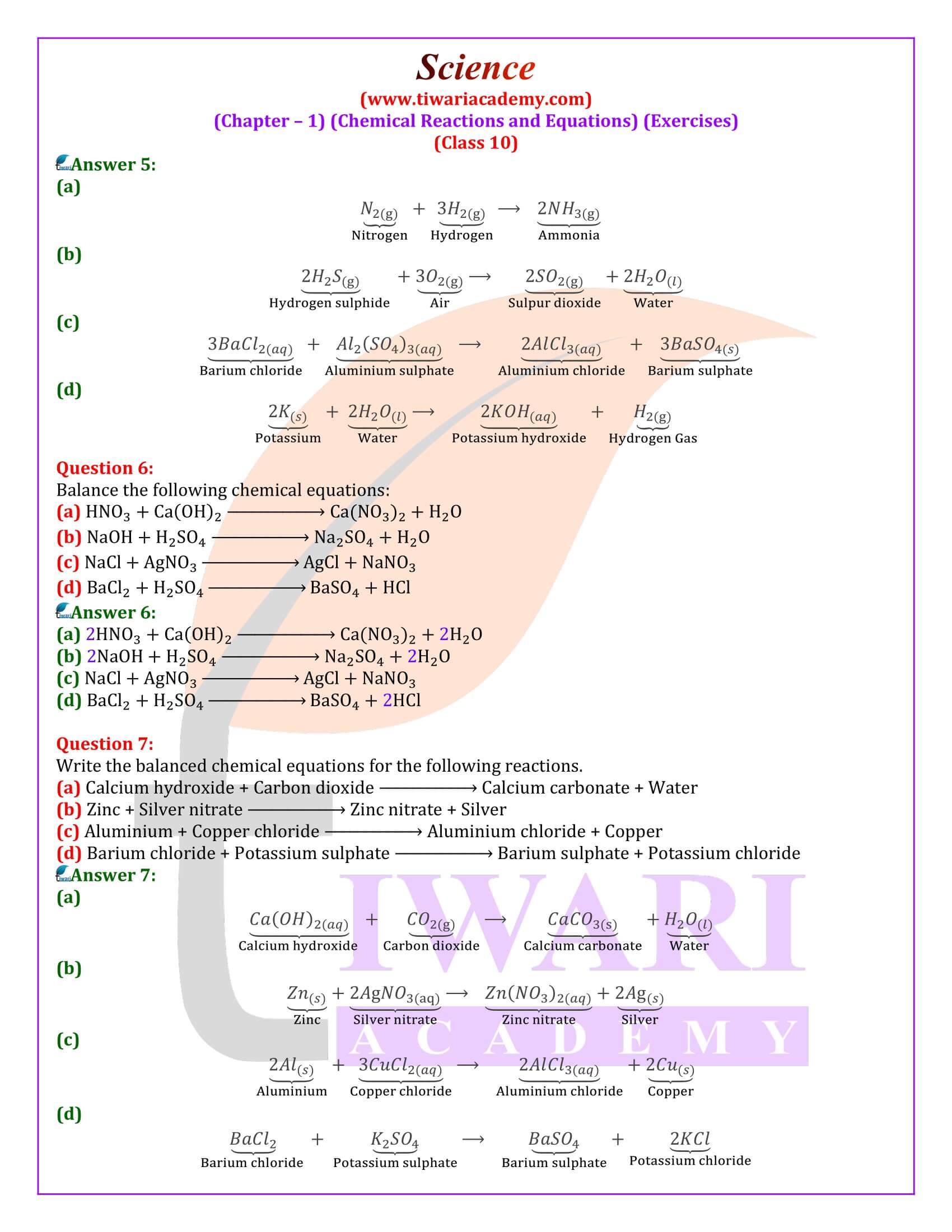
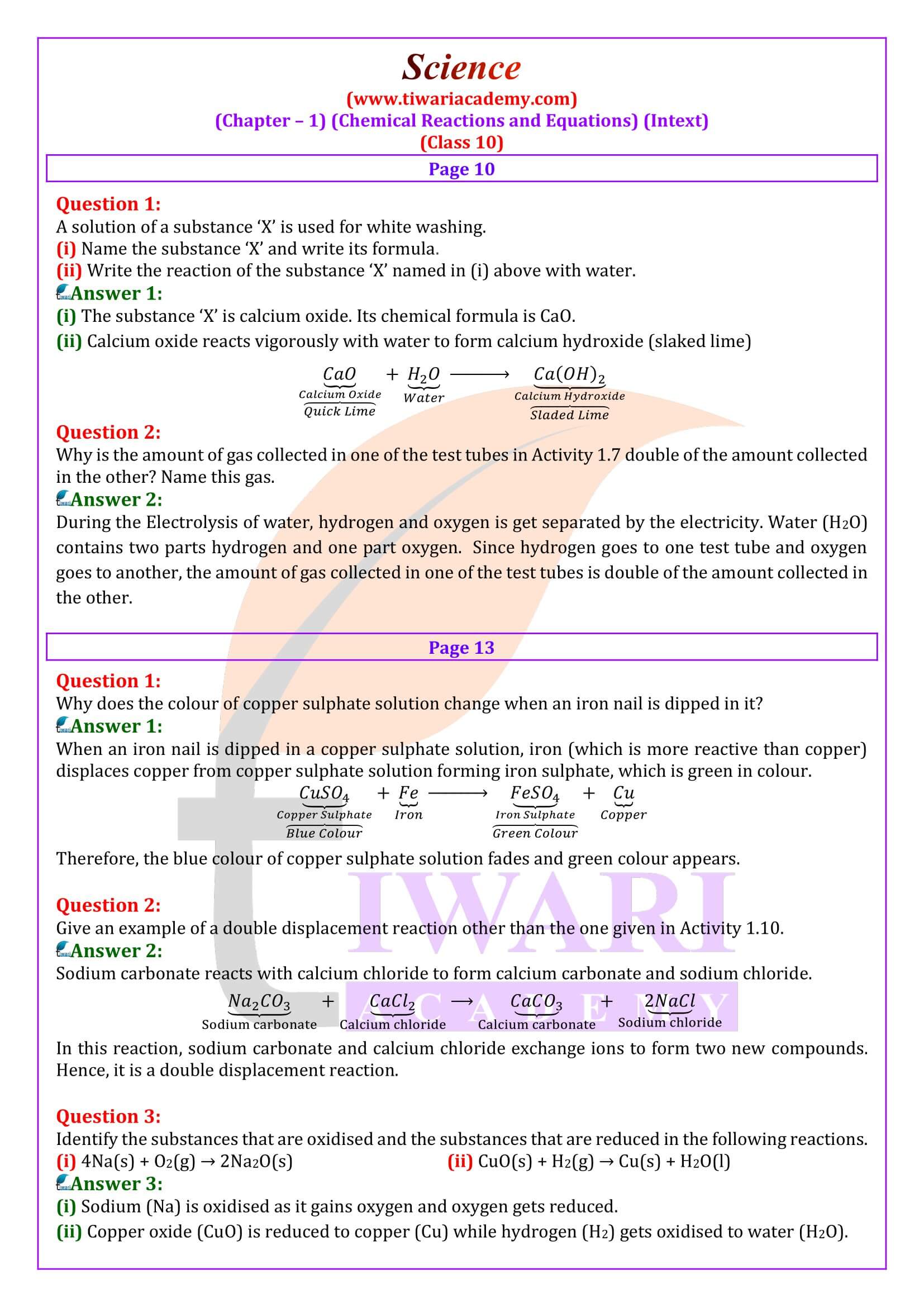
Question 2:
Explain the following terms with examples in each case:
(i) Oxidation and (ii) Reduction.
Answer 2:
(i) Oxidation: it us a process (a) in which oxygen or any electronegative element is added up or (b) hydrogen or any electropositive element is removed. Examples are:
(a)Sulphur burns in the air with a blue flame to form sulphur dioxide. Here oxygen is added up to sulphur.
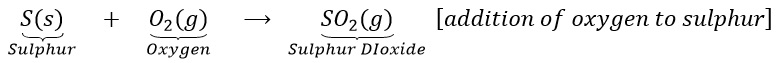
(b) Hydrogen sulphide combines with iodine to give hydrogen iodine and sulphur.
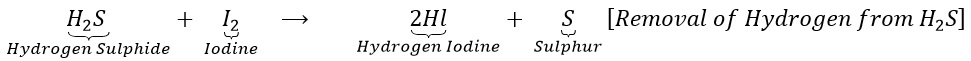
(ii) Reduction: It is a process in which (a) Hydrogen or an electropositive element is added up.
(b) Oxygen or electronegative element is removed.
Examples are:
(a)Hydrogen reacts with chlorine to form hydrogen chloride.
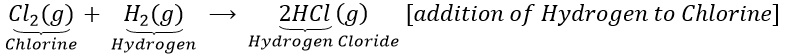
(b)Copper oxide is reduce with hydrogen.
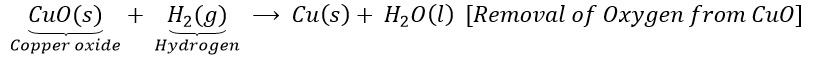
The chapter 1 of 10th Science include descriptions of simple experiments related to chemical reactions. Questions about experimental procedures and observations are often asked in exams to test students’ practical knowledge.
Since 10th Science Chapter 1 deals with fundamental concepts and equations, it offers good scoring potential. If students thoroughly understand the content and practice solving problems, they can score well in this chapter.
Concepts introduced in Class X Science chapter 1, such as balancing equations and identifying types of reactions, are applied in subsequent chapters of the Class 10 Science syllabus. A strong grasp of Chapter 1 is essential for success in these chapters as well.
In summary, NCERT Class 10 Science Chapter 1 is important not only for the foundational knowledge it provides but also because it sets the stage for understanding more complex chemistry concepts in higher classes. Students should give due attention to this chapter in their exam preparation to perform well in their Class 10 Science board exams.
10 Science – Chemical Reactions and Equations – Important Questions
Why is respiration considered an exothermic reaction?
Answer::
During digestion, food is broken down into simpler substances. Food like rice, potato and bread are made up of carbohydrates. These carbohydrates are further broken down into glucose. Glucose during respiration (inhalation of oxygen) is oxidised with the liberation of energy as shown below. Thus, respiration is an exothermic process.
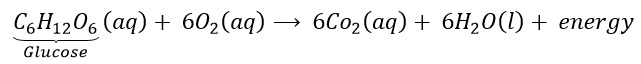
FAQ:
What are the main concepts to focus in chapter 1 Chemical Reactions and Equations of class 10th Science?
Concepts that students will study in chapter 1 (Chemical Reactions and Equations) of class 10th Science are:
- 1. Chemical Equations.
1.1 Writing a Chemical Equation
1.2 Balanced Chemical Equation - 2. Types of Chemical Reactions
2.1 Combination Reactions
2.2 Decomposition Reaction
2.3 Displacement Reaction
2.4 Double Displacement Reaction
2.5 Precipitation Reaction
2.6 Oxidation and Reduction - 3. Have you observed the effects of oxidation reactions in everyday life?
3.1 Corrosion
3.2 Rancidity
Is chapter 1 Chemical Reactions and Equations of Standard 10th Science challenging?
Chapter 1 Chemical Reactions and Equations of grade 10th Science is not at all challenging. This chapter is overall an easy chapter, but few students face problems while balancing the chemical equation. However, the difficulty level of any topic depends on students also. Some students find chapter 1 easy, and some students find chapter 1 difficult.
Is there any book other than NCERT for chapter 1 of class 10th Science that students can refer to at the time of exams?
Yes, there is a book other than NCERT for chapter 1 of class 10th Science that students can refer to at the exam time. The name of this book is NCERT Exemplar or Lakhmir Singh and Manjit Kaur. These books are one of the best books after NCERT textbook. The language of books is students friendly. Students can easily prepare chapter 1 of class 10th Science from these books.
How much time is needed to complete chapter 1 of 10th Science NCERT Book?
Students need a maximum of 8-10 days to complete chapter 1 of class 10th Science if they give 1-2 hours per day to this chapter. This time is an approximate time. This time can vary because no students can have the same speed, efficiency, ability, etc.
Which substances can be spoil due to corrosion according to chapter 1 of class 10th Science?
Corrosion causes damage to bodies of cars, bridges, railings made up of iron, ships, and to all objects made of metals, especially those of iron. Corrosion of iron is a serious problem. Every year a large amount of money is spent to replace damaged iron.
What do you mean by oxidation and reduction in chapter 1 of class 10th Science?
Oxidation: It is the gain of oxygen or loss of hydrogen.
Reduction: It is the loss of oxygen or the gain of hydrogen.
How many questions are there in chapter 1 of class 10th Science Book?
There are 28 questions in chapter 1 of class 10th Science. 8 questions are in between the chapter, and 20 questions are in the back exercise of the chapter. All questions are important. Students should do all questions of this chapter for the board exams.