Class 10 Science Chapter 4 Question Answers
Class 10 Science Chapter 4 Exercises Solutions
Class 10 Science Chapter 4 Intext Exercises
Class 10 Science Chapter 4 in Hindi Medium
Class 10 Science Book Download in PDF
Class 10 Science Chapter 4 Board Questions
Class 10 Science Chapter 4 MCQ
Class 10 Science Chapter 4 Extra Questions
Class 10 Science NCERT Solutions
Class 10 all Subjects Solutions
NCERT Solutions for Class 10 Science Chapter 4 Carbon and its compounds in Hindi and English Medium for CBSE 2024-25 board exam. The question answers and solutions of chapter 4 class 10th science is revised according to syllabus and new books issued for academic year 2024-25. In NCERT Class 10 Science, Chapter 4 is titled Carbon and Its Compounds.
Class 10 Science chapter 4 explores various aspects of carbon chemistry and the compounds formed by carbon atoms. Below is an outline of the topics covered in NCERT Class 10 Science Chapter 4. Here we learn the introduction to Carbon Compounds.
| Class: 10 | Science |
| Chapter 4: | Carbon and its compounds |
| Contents: | Intext and Exercise Solutions |
| Content Type: | PDF and Videos Format |
| Session: | CBSE 2024-25 |
| Medium: | English and Hindi Medium |
Class 10 Science Chapter 4 Answers in Hindi and English Medium
- Class 10 Science Chapter 4 Exercises
- Class 10 Science Chapter 4 Intext Questions
- Class 10 Science Chapter 4 in Hindi
- Class 10 Science Chapter 4 in PDF
- Class 10 Science Chapter 4 Board Questions
- Class 10 Science Chapter 4 MCQ
- Class 10 Science Chapter 4 Extra Questions
- Class 10 Science NCERT Solutions
- Class 10 all Subjects Solutions
Explanation of the unique properties of carbon and its ability to form a vast number of compounds. Covalent Bonding in Carbon Compounds. Understanding the concept of covalent bonding and its role in the formation of carbon compounds.

Saturated and Unsaturated Carbon Compounds
Differentiating between saturated and unsaturated hydrocarbons. Explanation of single, double, and triple bonds in carbon compounds. Discussion on the versatility of carbon in forming chains, branched structures, and rings, leading to the creation of a diverse range of organic compounds. Introduction to functional groups and their significance in organic chemistry.
Homologous Series and Nomenclature of Carbon Compounds
Explanation of the concept of a homologous series and the characteristics it exhibits. Examples of homologous series, such as alkanes, alkenes, and alkynes. Name of the compound under the IUPAC system. Examples of naming simple organic compounds. Understanding the concept of isomerism, where compounds have the same molecular formula but different structural arrangements. Examples of isomers in organic compounds.
NCERT Solutions for Class 10 Science Chapter 4
Class X Science chapter 4 intext questions given on Page 61 or Page 68 or Page 71 or Page 74 or Page 76 or Exercises in English updated for academic session 2024-25. Download all the pages and Abhyas in Hindi Medium also to view online or download in PDF format free for new academic session. Download CBSE Solutions Apps based on latest CBSE Syllabus for offline use.
Chemical Properties of Acids and Bases
Basic properties of acids and bases. Introduction to the concept of pH scale. Reaction of acids with various compounds. Reactions of bases with non-metals like carbon dioxide. Neutralization reactions between acids and bases. Practical applications of carbon compounds in various industries and daily life. These are the main topics typically covered in NCERT Class 10 Science Chapter 4, Carbon and Its Compounds.
Chemical Properties of Carbon Compounds
Reactions of carbon compounds with oxygen, including combustion. Reactions with hydrogen and halogens. Introduction to the concept of addition reactions. Detailed discussion of properties and uses of ethanol and ethanoic acid (acetic acid). Explanation of the structure and properties of soaps and detergents. How soaps and detergents work to clean surfaces and remove dirt and stains. Understanding the cleansing action of soaps, including the formation of micelles.
Studying NCERT Class 10 Science Chapter 4, Carbon and Its Compounds, effectively can help you secure good marks in your exams. Here’s a step-by-step guide on how to study this chapter. Begin by reading the entire chapter from your NCERT textbook. Pay close attention to the explanations, examples, and diagrams provided in the book. Understand the basic concepts and the importance of carbon compounds.
Grasp the concept of isomerism, where compounds have the same molecular formula but different structural arrangements. Practice identifying isomers to reinforce your understanding. Pay special attention to the chemical reactions involving carbon compounds. Understand the reactions with oxygen (combustion), hydrogen, halogens, and other elements. This chapter may include numerical problems related to chemical reactions and properties of carbon compounds. Practice solving these problems to build your problem-solving skills.
As you read, take clear and concise notes. Summarize key points, definitions, and chemical reactions. Prepare notes in a proper way for easy reference. Carbon compounds are based on covalent bonding. Ensure you have a solid understanding of how covalent bonds work and how they contribute to the formation of organic compounds. Understand the IUPAC system for naming organic compounds. Practice naming various carbon compounds following the rules of nomenclature.
Understand the role of functional groups in organic compounds. Familiarize yourself with common functional groups like alcohols, acids, and esters. These compounds are frequently asked about in exams. Learn their properties, chemical reactions, and uses. Understand the structure and properties of soaps and detergents. Learn how they work as cleansing agents and their advantages over each other.
Class 10 Science Chapter 4 Extra Question Answers
Why are covalent compounds poor conductor of electricity?
Covalent compounds are formed due to sharing of electrons between atoms and no charged particle (ions) are formed, hence such compounds are generally bad conductors of electricity.
Differentiate between saturated and unsaturated hydrocarbons.
Saturated hydrocarbons:
A hydrocarbon in which each carbon atom is attached to four other atoms, is known a saturated hydrocarbon. The bonds so formed are single covalent bonds. These hydrocarbons are also called alkanes.
Unsaturated Hydrocarbons:
Hydrocarbons contains either a carbon-carbon double bond (C = C) or a carbon-carbon triple bond in their molecules are called unsaturated hydrocarbons.
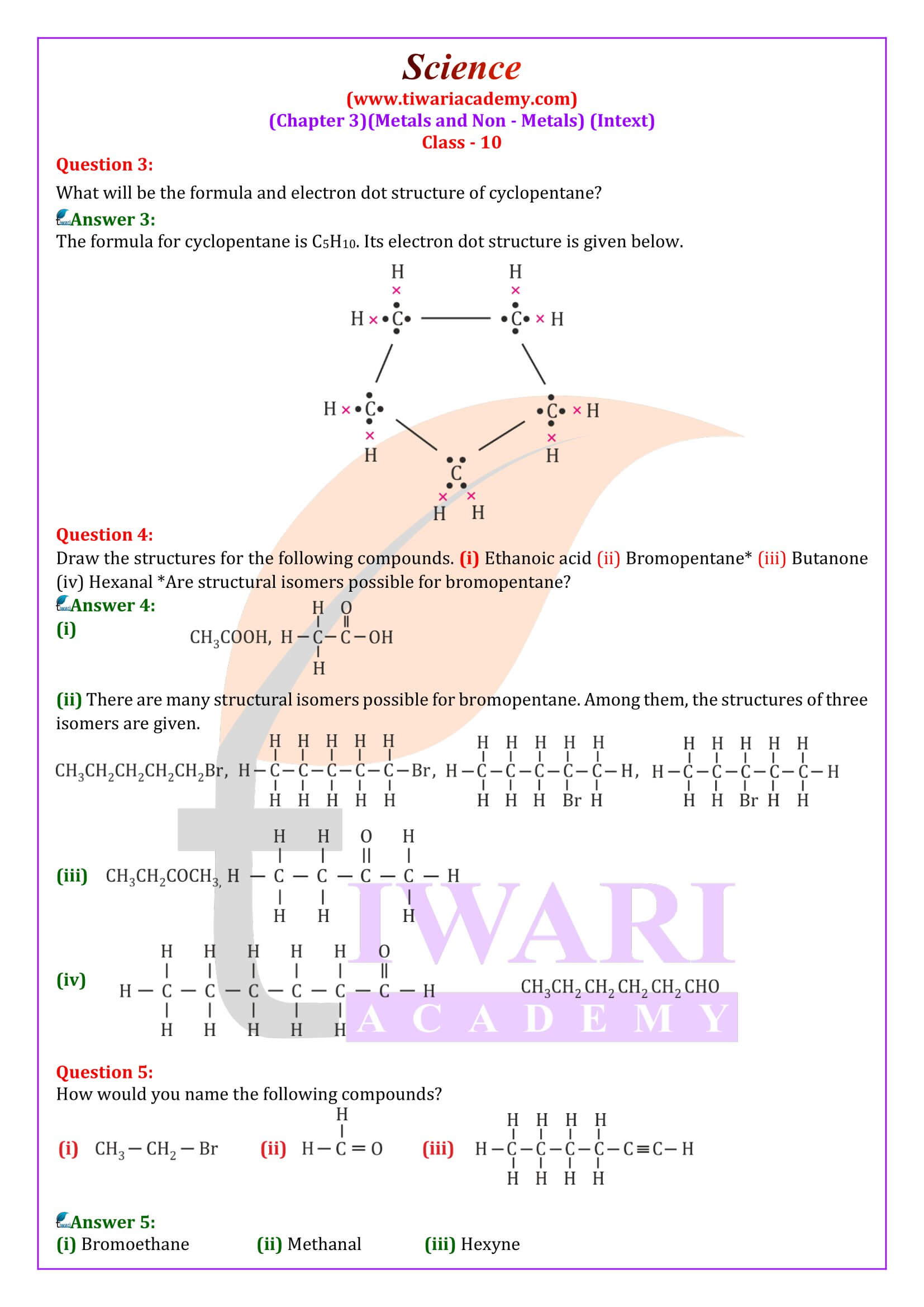
What is catenation? What is its property?
The unique property of carbon to form bonds with other carbon atoms giving rise to large molecules is called catenation.
Due to catenation compounds of carbons have long chains of carbon, branches chains of carbon and even carbon atoms arranged in rings.
What is meant by saponification?
The reaction of an ester to react with an acid or base to give back the alcohol and carboxylic acid is called saponification. This is so-called because this reaction is used in the preparation of soap.
How does soap help to wash the clothes?
Dirt is generally absorbed in the clothes as an oily material. It cannot be removed with water because it does not mix well with water. But when a cloth with dirt is soaked in soap solution, the dirt and grease attach themselves to the hydrocarbon component of the soap molecule. The ― COONa part of the soap which is attached to the water molecules pulls the hydrocarbon part along with dirt away from the surface of the cloth, thus washing it clean.
Chemical Properties of Acids and Bases
Understand the chemical properties of acids and bases, including their reactions with different substances. Learn how to calculate pH values and understand the concept of neutralization. Solve sample papers and previous years’ question papers to get a sense of the exam pattern and practice answering questions within the allotted time. Explore online resources, educational websites, and video tutorials that cover the topics of Chapter 4 of 10th Science in different ways. These can provide additional explanations and examples.
10th Science Chapter 4 Answers in English & Hindi Medium
NCERT solutions for class 10 Science all chapters based on latest NCERT Books are in the same format. You can use these solutions for online study or download to use it offline. 10 Science Chapter 4 all intext questions and chapter end exercises question answers are given below. Periodically test your knowledge by taking self-assessment quizzes and mock tests related to Chapter 4. Identify areas where you need further practice.
If you have doubts or questions about any topic within the chapter, don’t hesitate to ask your teacher or seek help from peers or online forums. As your exams approach, revise the chapter thoroughly. Focus on your notes, important formulas, chemical reactions, and frequently asked questions. By following these study strategies and maintaining a consistent study routine, you can prepare effectively for NCERT Class 10 Science Chapter 4 and increase your chances of scoring well in the examination.
To prepare well for NCERT Class 10 Science Chapter 4, “Carbon and Its Compounds,” through Tiwari Academy, you can follow these steps. Tiwari Academy typically provides detailed NCERT solutions for Class 10 Science Chapter 4. Begin by using these solutions to understand the concepts and solve the textbook exercises.
Tiwari Academy may offer video lessons or tutorials related to Chapter 4. Video lessons can provide a visual and interactive way to understand complex concepts and chemical reactions. Tiwari Academy often offers a variety of practice questions and exercises related to the chapter. Work through these questions to reinforce your understanding and problem-solving skills.
Important Questions on 10th Science Chapter 4
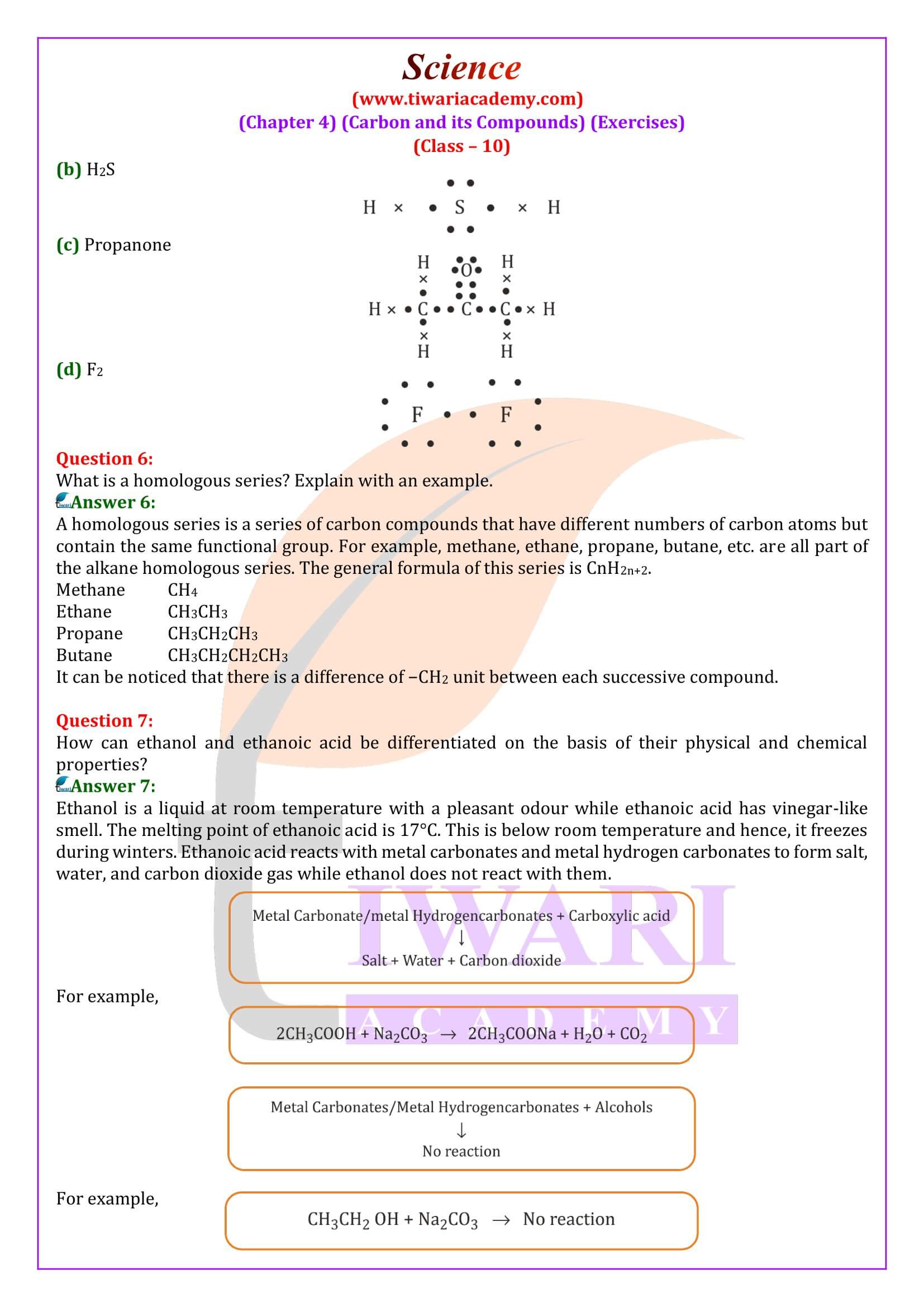
What is a homologous series?
A homologous series is a series of carbon compounds that have different numbers of carbon atoms but contain the same functional group. For example, methane, ethane, propane, butane, etc. are all part of the alkane homologous series. The general formula of this series is CnH2n+2.
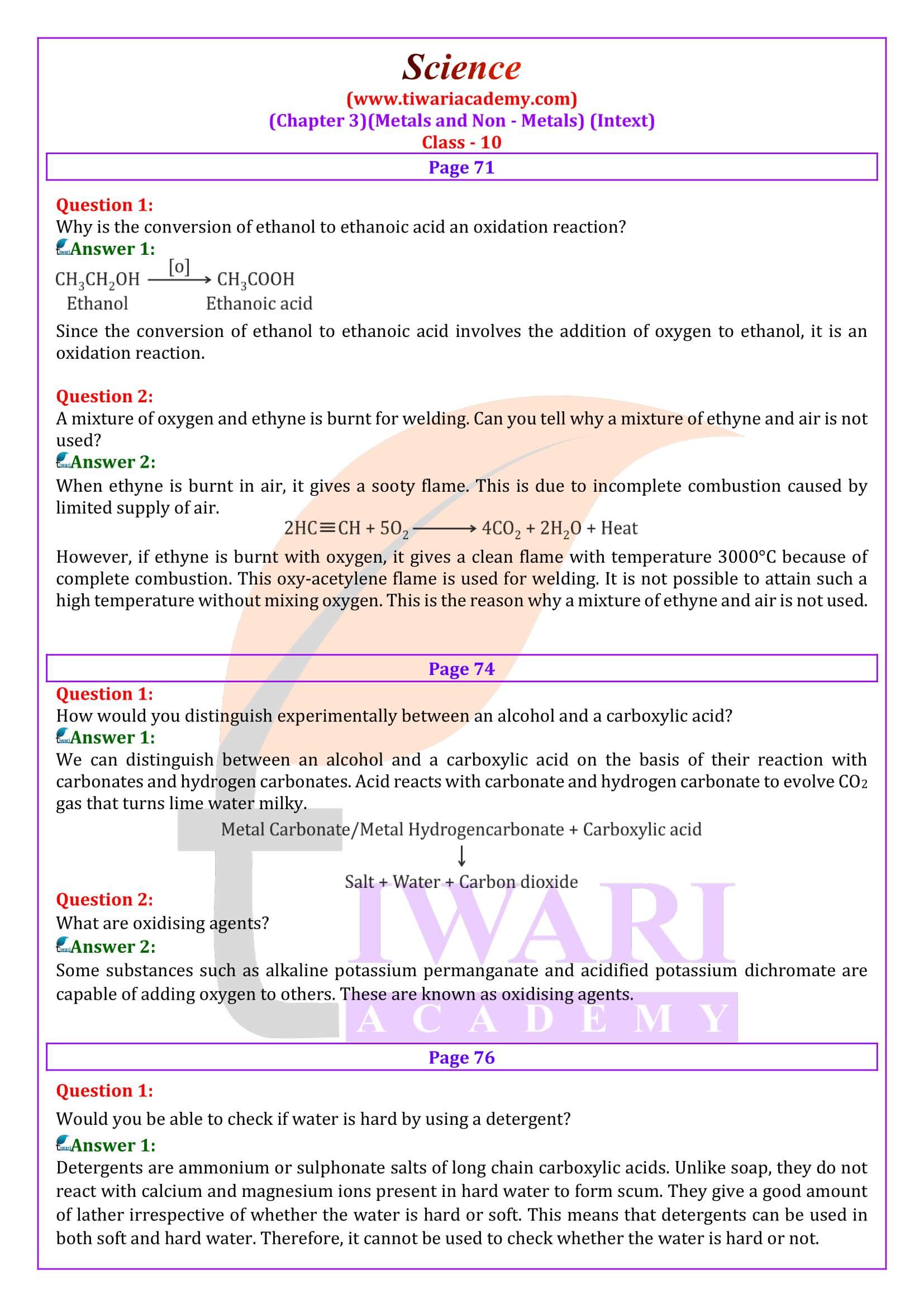
How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Ethanol is a liquid at room temperature with a pleasant odour while ethanoic acid has vinegar-like smell. The melting point of ethanoic acid is 17°C. This is below room temperature and hence, it freezes during winters. Ethanoic acid reacts with metal carbonates and metal hydrogencarbonates to form salt, water, and carbon dioxide gas while ethanol does not react with them.
Why are carbon and its compounds used as fuels for most applications?
Most of the carbon compounds give a lot of heat and light when burnt in air. Saturated hydrocarbons burn with a clean flame and no smoke is produced. The carbon compounds, used as a fuel, have high calorific values. Therefore, carbon and its compounds are used as fuels for most applications.
Explain the formation of scum when hard water is treated with soap.
Soap does not work properly when the water is hard. A soap is a sodium or potassium salt of long chain fatty acids. Hard water contains salts of calcium and magnesium. When soap is added to hard water, calcium and magnesium ions present in water displace sodium or potassium ions from the soap molecules forming an insoluble substance called scum. A lot of soap is wasted in the process.
What change will you observe if you test soap with litmus paper (red and blue)?
Since soap is basic in nature, it will turn red litmus blue. However, the colour of blue litmus will remain blue.
What is hydrogenation? What is its industrial application?
Hydrogenation is the process of addition of hydrogen. Unsaturated hydrocarbons are added with hydrogen in the presence of palladium and nickel catalysts to give saturated hydrocarbons. This reaction is applied in the hydrogenation of vegetables oils, which contain long chains of unsaturated carbons.
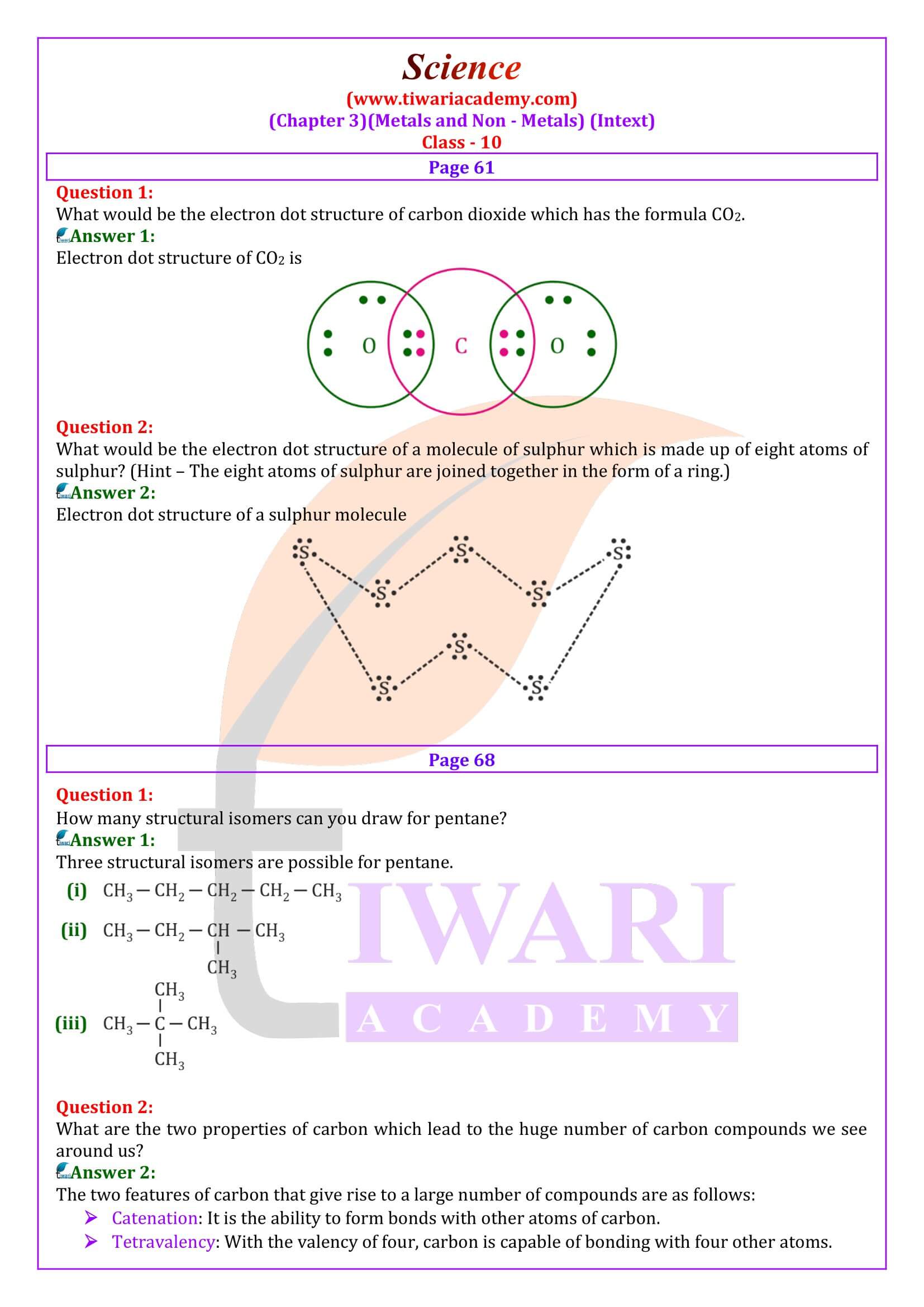
What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
The two features of carbon that give rise to a large number of compounds are as follows: (i) Catenation: It is the ability to form bonds with other atoms of carbon. (ii) Tetravalency: With the valency of four, carbon is capable of bonding with four other atoms.
What are oxidising agents?
Some substances such as alkaline potassium permanganate and acidified potassium dichromate are capable of adding oxygen to others. These are known as oxidising agents.
Create flashcards for key terms, definitions, and chemical reactions. Flashcards are a useful tool for quick revision and self-assessment. Take advantage of online tests and quizzes provided by Tiwari Academy. These tests can help you assess your knowledge and track your progress. Tiwari Academy may provide sample papers and previous year’s question papers for Class 10 Science.
Practice solving these papers to get a feel for the exam pattern and improve your time management. Explore any interactive features offered by Tiwari Academy, such as forums, discussion boards, or chat support. These platforms can allow you to interact with teachers and peers, fostering a collaborative learning environment.
Questions for Practice
Question 1:
What do you understand by a homologous series? Explain giving one example of homologous series.
OR
Define homologous series of carbon compound. List any two characteristics of a homologous series.
Answer 1:
A homologous series is a group or family of compounds which contains the same functional group but have different chain lengths. Thus, these have the same chemical properties but different physical properties that vary in a regular manner.
Characteristics of a homologous series are:
(i) It has a general formula in terms of number of carbon atoms.
(ii) It has the same functional group, if any.
(iii) The members of a homologous series, i.e., homologous, have similar chemical properties.
(iv) Various homologous can be prepared by the general method of preparation for the series.
(v) Two successive (adjacent) homologous differ by 1 carbon atom and 2 hydrogen atoms in their molecular formulae.
(vi) The member of a homologous series show a gradual change in their physical properties with increase in molecular mass.
Question 2:
(a) Distinguish between ethanol and ethanoic acid on the basis of
(i) litmus test, (ii) reaction with sodium hydrogen carbonate.
(b) Name the oxidising agents used in the conversion of ethanol to ethanoic acid.
Answer 2:
(a) Ethanol shows no change/reaction with either litmus paper or sodium hydrogen carbon.
Ethanoic acid solution in water turns blue litmus red. It reacts with sodium carbonate with effervescence and gives out carbon dioxide gas which turns lime water milky.
(b) Alkaline potassium permanganate turns ethanol to acid.
If you have doubts or questions about any topic within Chapter 4 of 10th Science, take advantage of Tiwari Academy’s doubt-clearing support. You may be able to ask questions and seek clarification on specific concepts. Tiwari Academy may provide tips and strategies for effective time management during exam preparation, helping you balance your study schedule with other activities. Explore any additional resources, animations, diagrams, and illustrations that Tiwari Academy offers to enhance your understanding of complex topics in Chapter 4.
Maintain a consistent study routine and revisit the chapter regularly for revision. Consistency is key to mastering the concepts. By utilizing these resources and strategies provided by Tiwari Academy, you can prepare effectively for NCERT Class 10 Science Chapter 4 and increase your chances of performing well in the examination. Remember to complement your online resources with your school textbook and regular study routines.
Why do soaps not work in hard water?
Question:
Soap does not form lather with hard water. Why?
Or
Why do soaps from scum instead of lather in hard water?
Answer:
When soap is added to a sample of hard water, calcium and/or magnesium ions present in hard react with soap forming insoluble calcium/magnesium soap which is a sticky and greasy mass and thus no lather is formed.
NCERT Class 10 Science Chapter 4, Carbon and Its Compounds, is significant from the perspective of board exams for several reasons. Questions related to this chapter typically carry a substantial weightage in the Class 10 Science board exams. Scoring well in Chapter 4 can significantly contribute to your overall science score.
Class 10 Science Chapter 4 introduces fundamental concepts related to organic chemistry, including covalent bonding, isomerism, and nomenclature. A strong understanding of these concepts is crucial as they serve as the foundation for more advanced organic chemistry topics in higher classes.
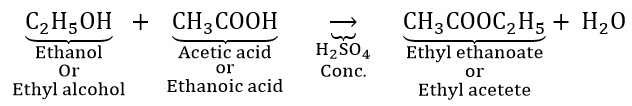
What will happen if ethanol reacts will ethanoic acid in the presence of an acid? Name the reaction. Write the chemical equation for the reaction.
Ethyl alcohol reacts with acetic acid (ethanoic acid) in the presence of a few drops of conc. sulphuring acid to form a sweet smelling substance called ester (ethyl acetate). Such a reaction is called esterification.
The chapter often includes numerical problems related to chemical reactions and properties of carbon compounds. Solving these problems effectively requires a good grasp of the concepts and problem-solving skills. Understanding the chemical reactions of carbon compounds, including combustion and other reactions, is essential. Questions related to these reactions are common in board exams. Knowledge of functional groups and their significance in organic chemistry is tested. This includes understanding the structures and properties of various functional groups.
The IUPAC system for naming organic compounds is an important part of the chapter. Questions related to naming compounds may appear in the exams. The chapter discusses practical applications of carbon compounds in various industries and daily life, including the uses of ethanol, ethanoic acid, and soaps. Questions related to these applications may be asked in the exams. The chapter also covers the properties of acids and bases and their chemical reactions. Understanding these concepts is important for a well-rounded knowledge of chemistry.
Some portions of the chapter discuss environmental aspects of carbon compounds, including issues related to pollution. These topics are relevant and may be tested in the context of environmental science.
In summary, NCERT Class 10 Science Chapter 4 is important for board exams because it covers foundational concepts in organic chemistry, chemical reactions, nomenclature, and practical applications of carbon compounds. Scoring well in this chapter can contribute significantly to your overall science score and provide a solid foundation for future studies in chemistry. Therefore, it is advisable to dedicate sufficient time and effort to prepare for this chapter thoroughly.
How many questions are there in chapter 4 of grade 10th Science NCERT Solutions?
There are 28 questions in chapter 4 of class 10th Science. 13 questions are in between the chapter, and 15 questions are in the back exercise of chapter 4. All questions are nice, interesting, and logical.
Which questions of chapter 4 of class 10th Science teacher are expected in the exams?
Chapter 4 of grade 10th Science is important from the exam point of view. Every year questions come from chapter 4 in the exams. There are 28 questions in chapter 4. All the questions of this chapter are significant and can come in the exams. But the most important questions of this chapter that teachers can give in the exams are questions 1, 2 (page number 61), questions 2, 3, 4 (page numbers 68, 69), question 1 (page number 71), question 1 (page number 74), question 2 (page number 76), and from back exercise questions 4, 5, 8, 11, 13, 14, 15 are important.
Is chapter 4 of class 10th Science practice lengthy?
Yes, chapter 4 of class 10th Science is lengthy. Students need a maximum of 13-15 days to complete chapter 4 (Carbon and its Compounds) of grade 10th Science if they give 1-2 hours per day to this chapter. This time depends on many factors like student’s working speed, efficiency, capability, etc.
Is chapter 4 of class 10th Science Solutions difficult to understand?
Yes, chapter 4 of class 10th Science is challenging. Most of the students face problems while solving the questions of this chapter and also in theory part of this chapter. This chapter is nice, interesting, and logical. This chapter requires complete concentration. Also, this chapter helps in increasing the thinking ability of students.