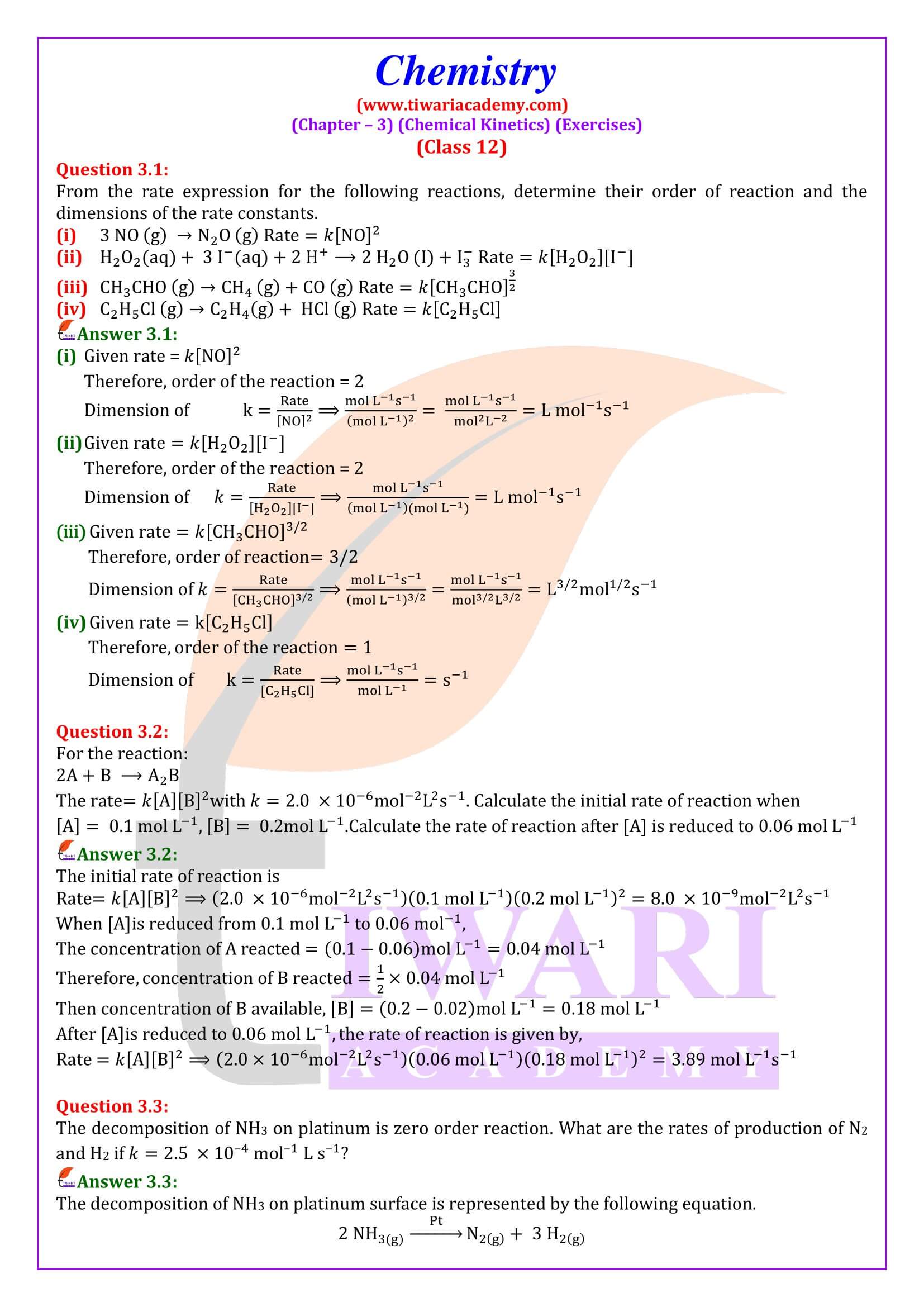
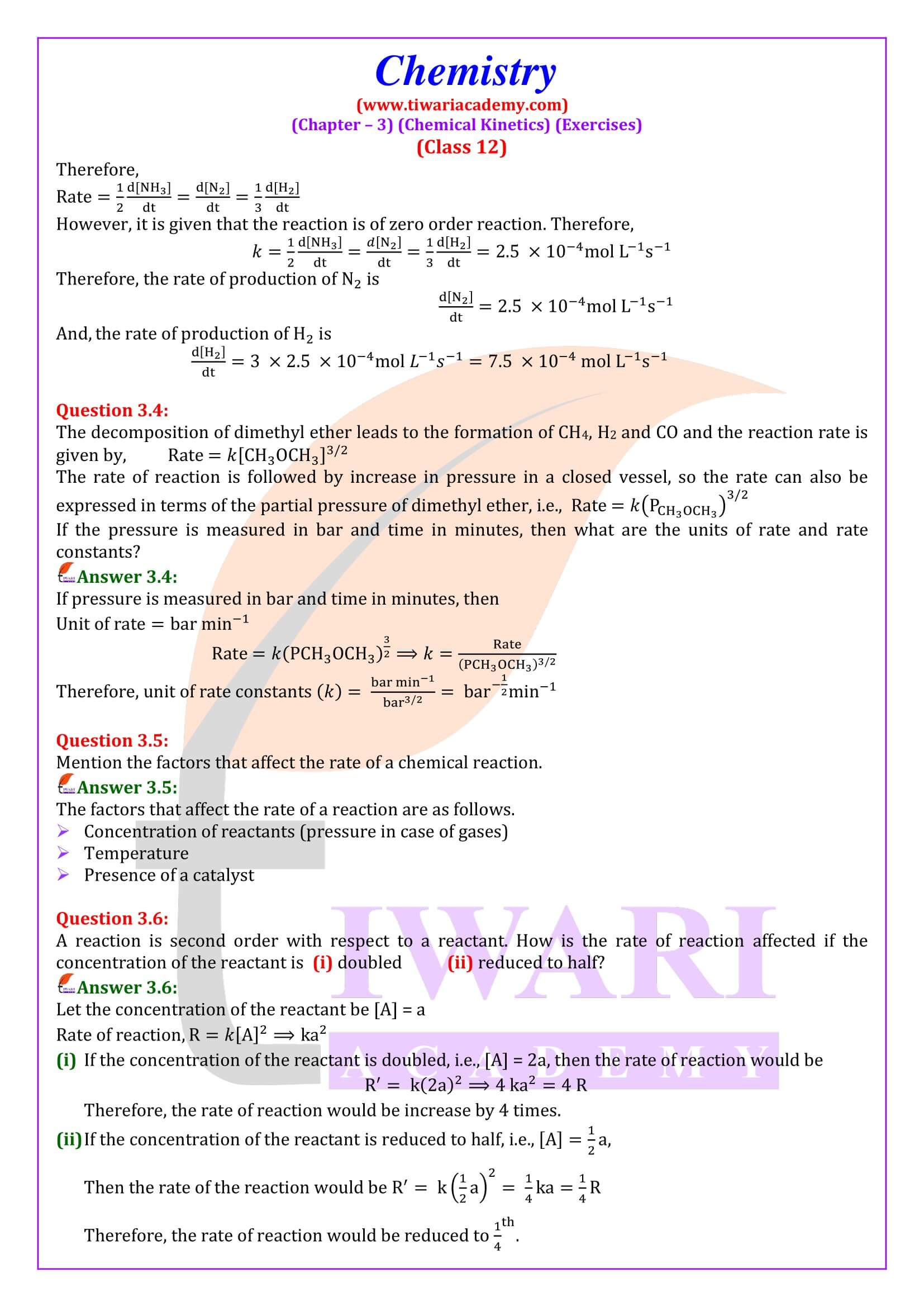
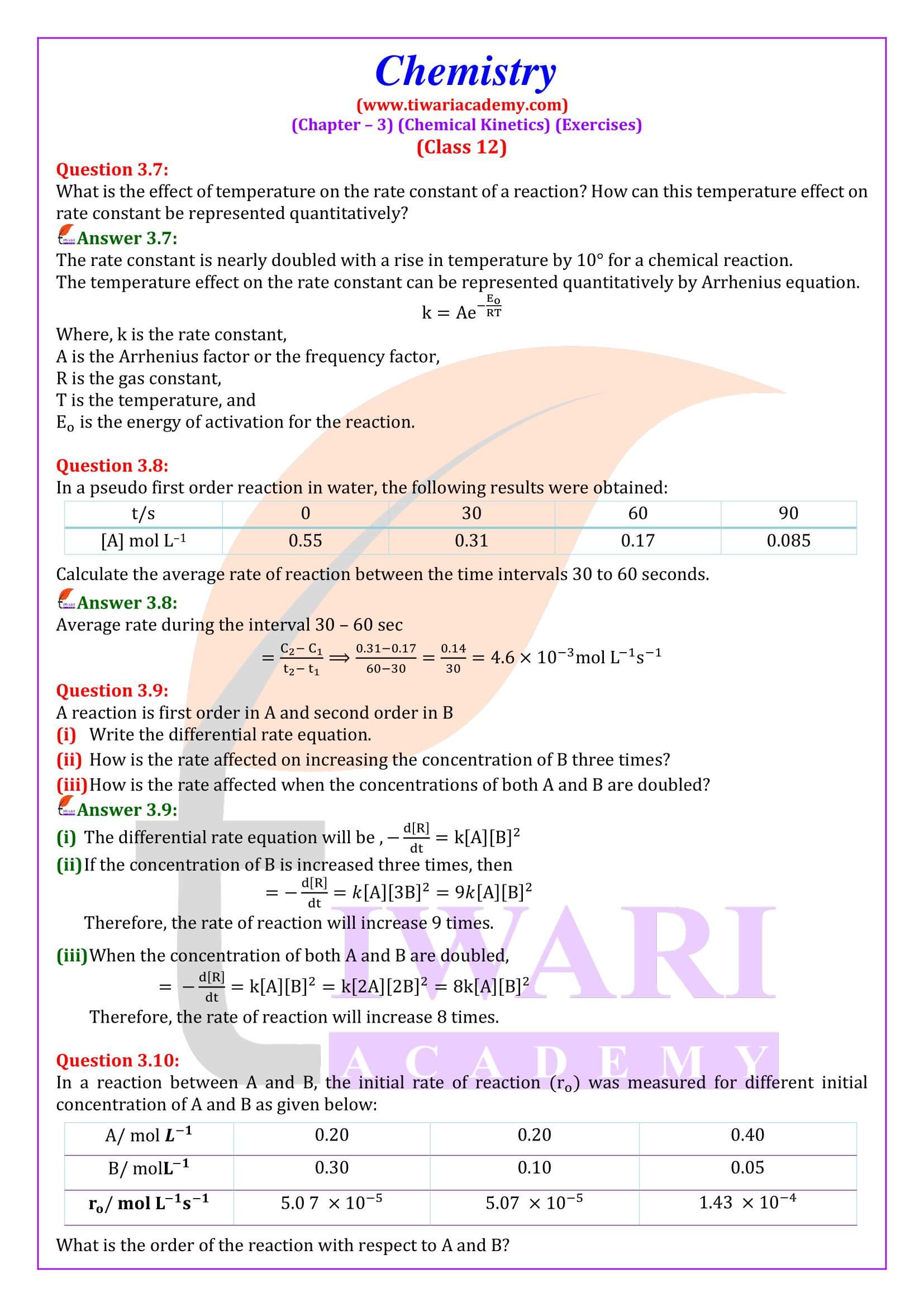
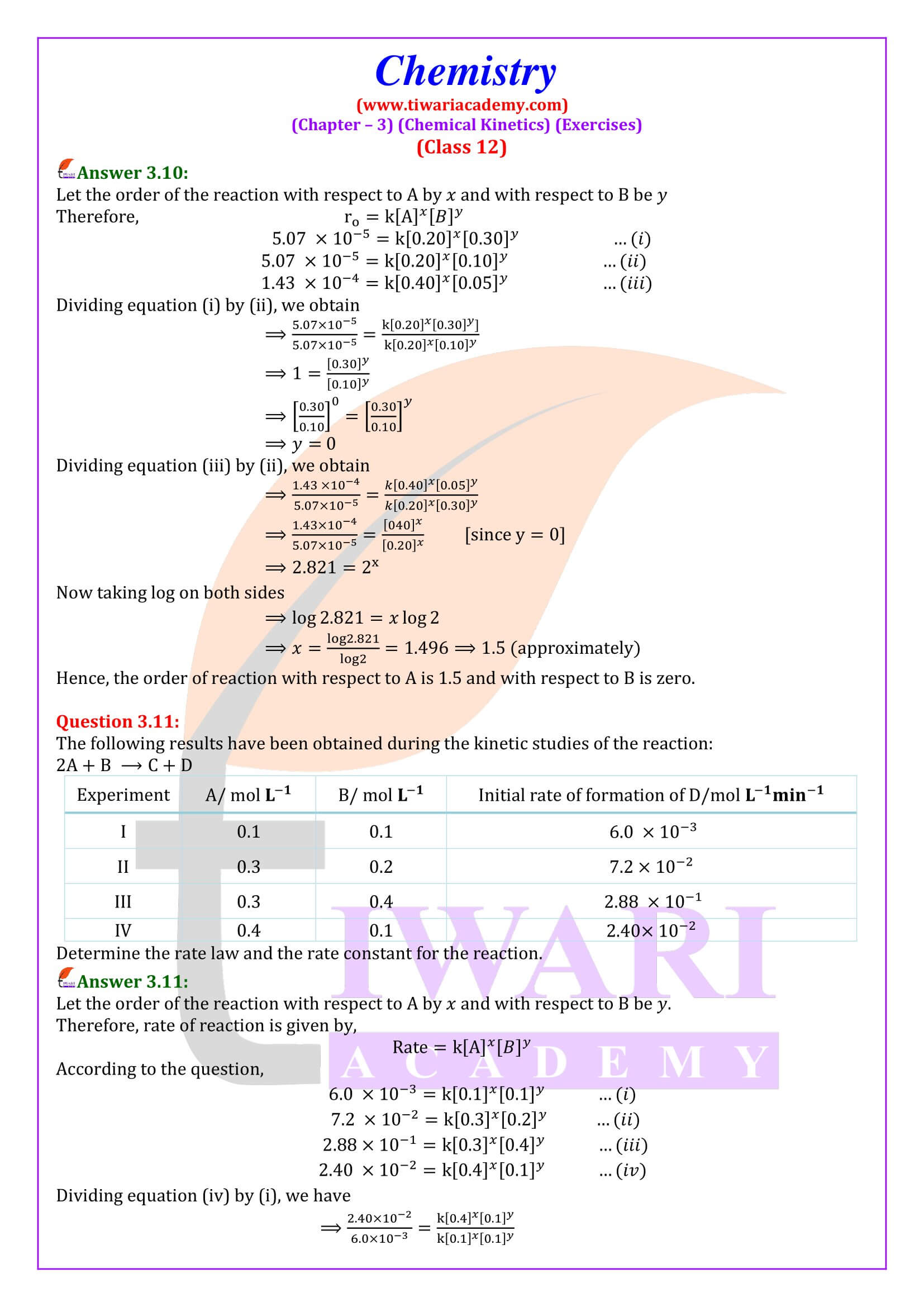
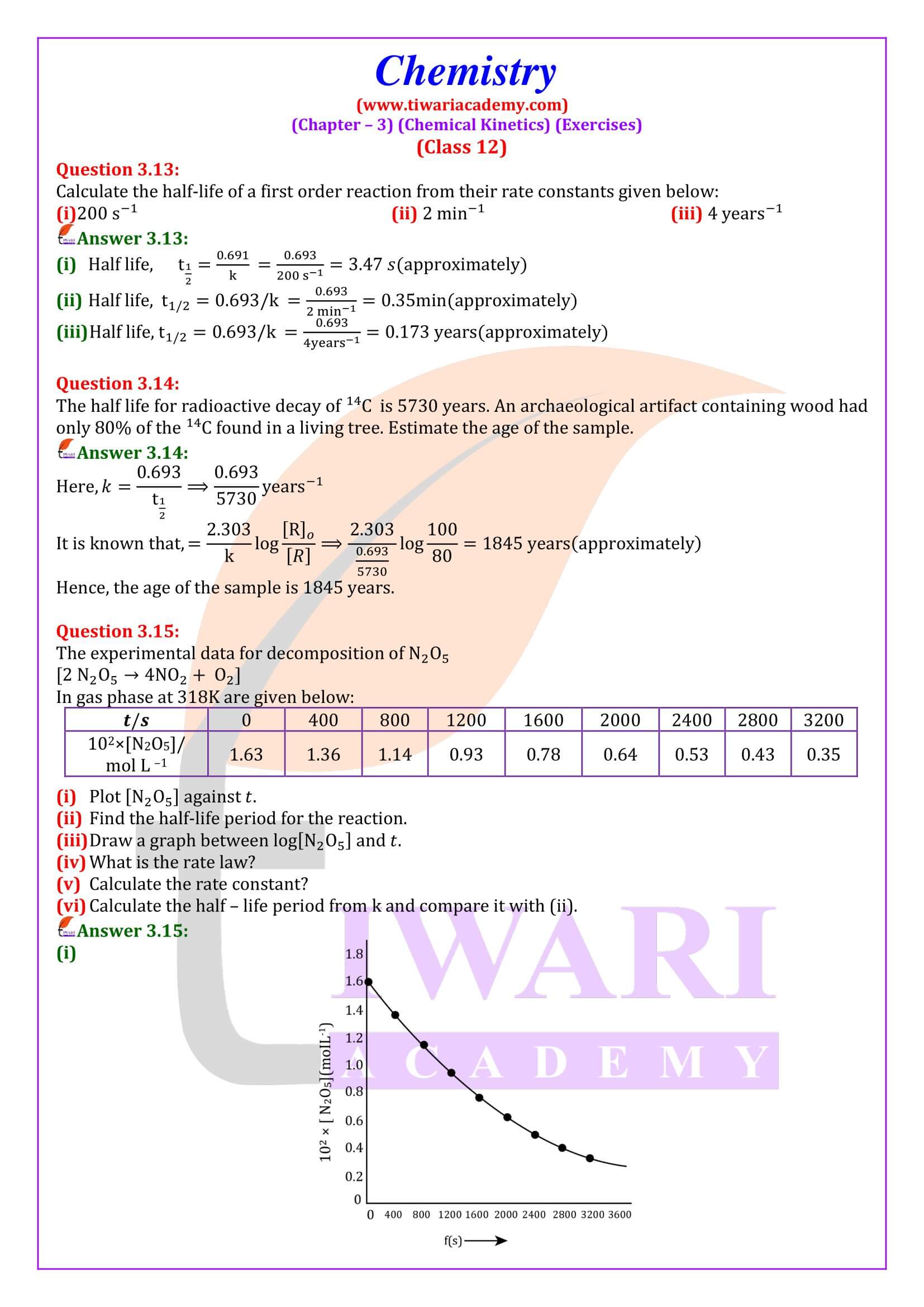
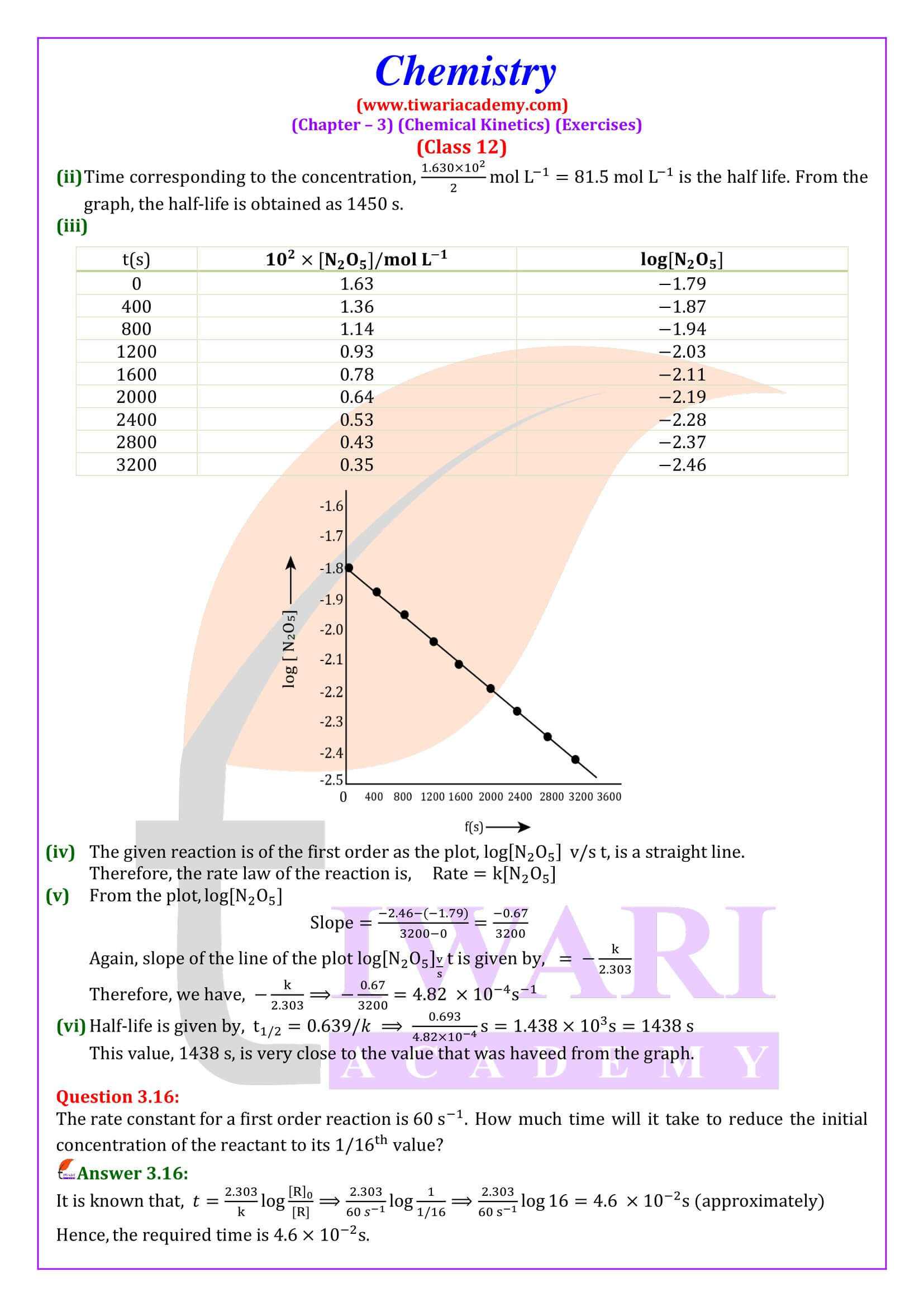
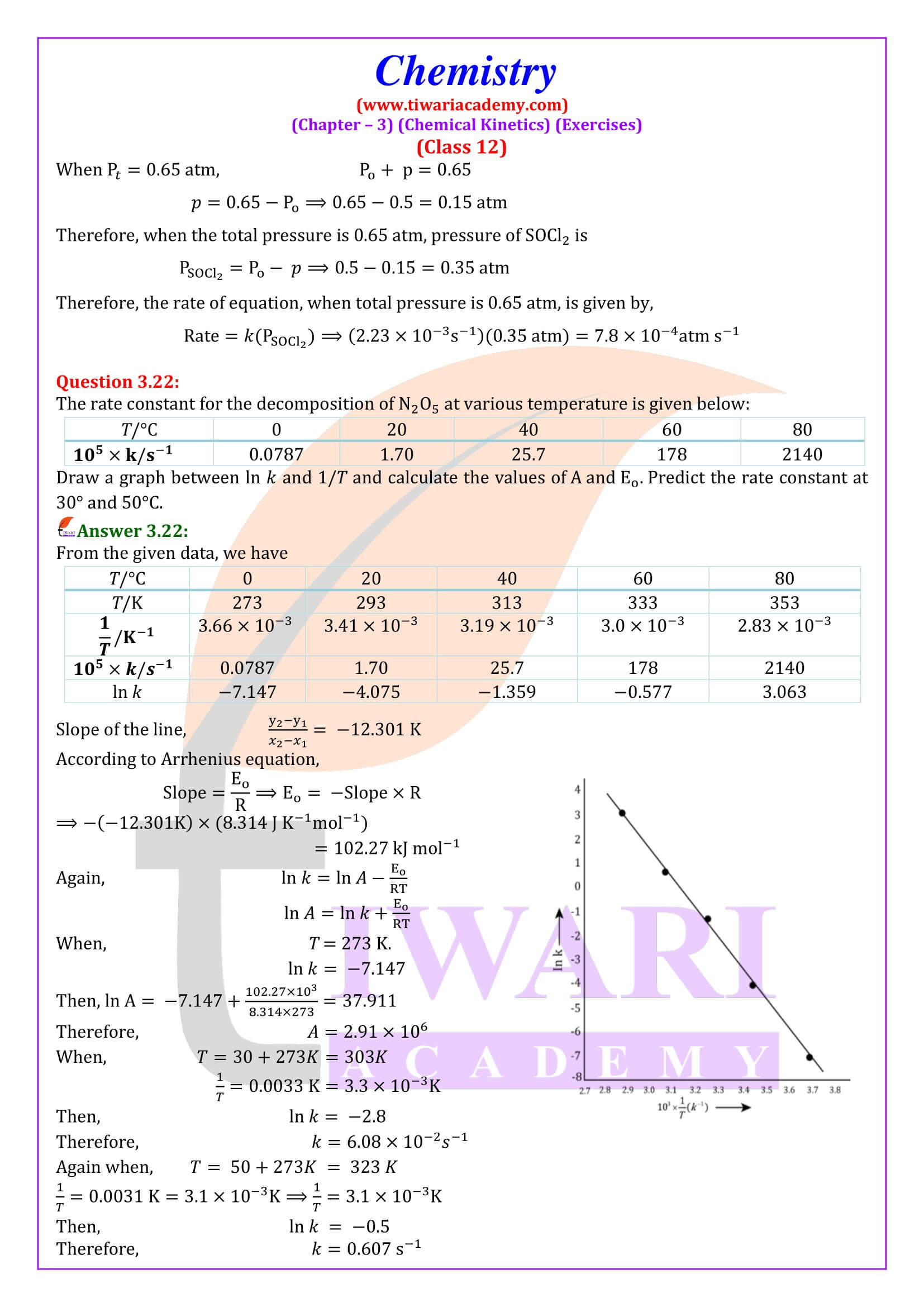
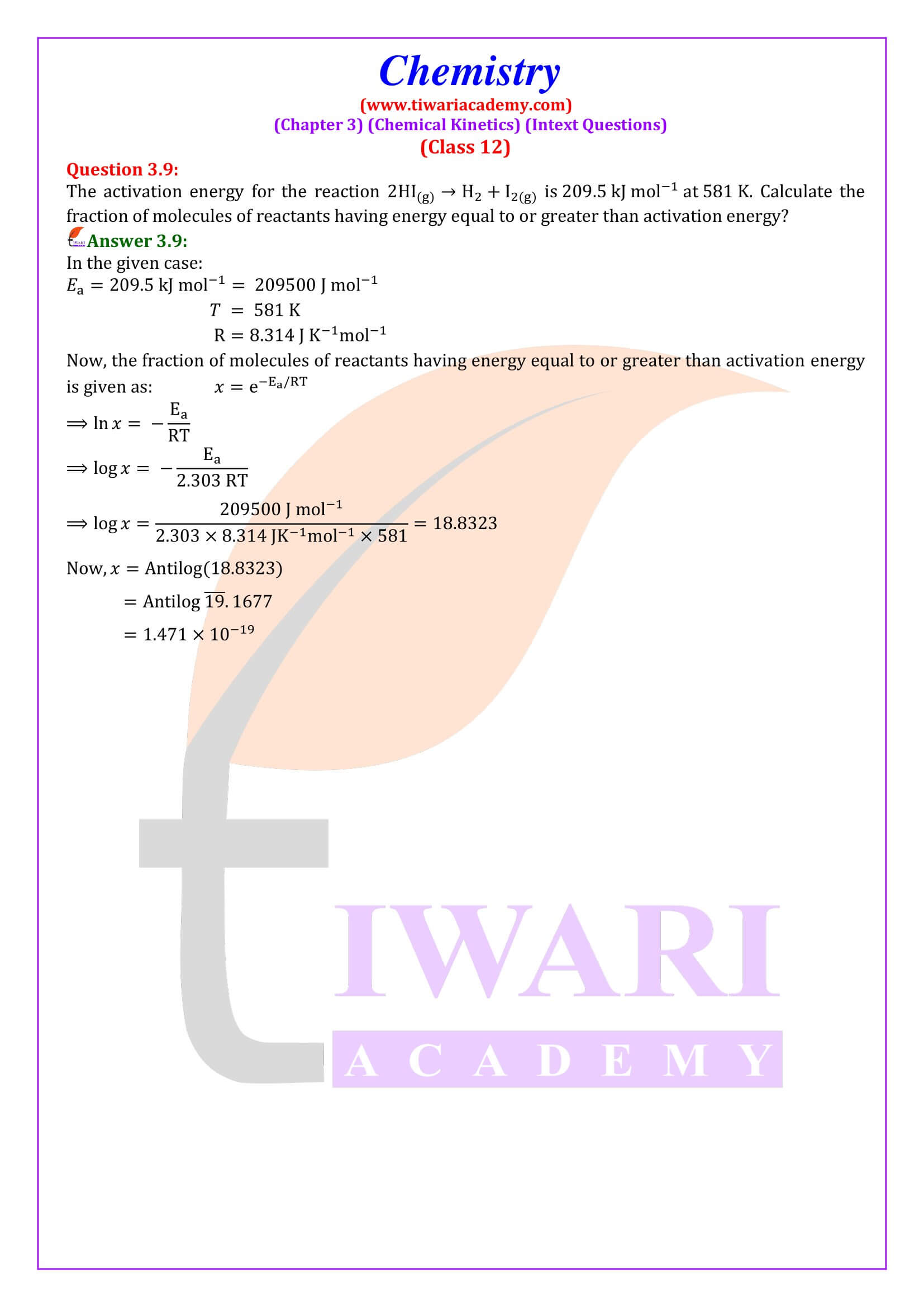
NCERT Solutions for Class 12 Chemistry Chapter 3 Chemical Kinetics in English and Hindi Medium updated for new academic session 2025-26. Class 12 Chemistry unit 3 intext questions and exercises are explained here in simplified format.
Practical Viva Questions for 12th Chemistry
NCERT Solutions for Class 12 Chemistry Chapter 3
Class 12 Chemistry Chapter 3 Chemical Kinetics Question Answers
| Class: 12 | Chemistry |
| Chapter: 3 | Chemical Kinetics |
| Content: | Exercises and Intext Question Answers |
| Content Format: | Images, Text and Videos |
| Academic Session: | Year 2025-26 |
| Medium: | Hindi and English Medium |
Chemical Kinetics
Chemical Kinetics is a branch of Chemistry which deals with chemical reaction, its factors and mechanism. It is closely related to the chemical reaction and physical process.
Rate of a Chemical Reaction
The speed at which a chemical reaction proceeds. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is formed in a unit of time or the concentration of a reactant that is consumed in a unit of time.
Consider a hypothetical reaction, assuming that the volume of the system remains constant.
R ⟶ P
One mole of the reactant R produces one mole of the product P. If [R]₁ and [P]₁ are the concentrations of R and P respectively at time t₁
and [R]₂ and [P]₂ are their concentrations at time t₂ then,
∆t = t₂ – t₁
∆[R] = [R]₂ – [R]₁
∆[P] = [P]₂ – [P]₁
The square brackets in the above expressions are used to express molar concentration.
Rate of disappearance of R = (Decrease in concentration of R) / (Time taken)
= – ∆[R] / ∆t …(1)
Rate of appearance of P = (Increase in concentration of P) / (Time taken)
= + ∆[P] / ∆t …(2)
Since, ∆[R] is a negative quantity (as concentration of reactants is decreasing), it is multiplied with –1 to make the rate of the reaction a positive quantity. Equations 1 and 2 given above represent the average rate of a reaction, rₐᵥ.
Class 12 Chemistry Chapter 3 MCQ
If the rate of a reaction is expressed by, rate = A [A]² [B], the order of reaction will be
The reaction of high molecularity are rare because
A reaction in which reactants (R) are converted into products (P) follows second order kinetics. If concentration of R is increased by four times, what will be the increase in the rate of formation of P?
Factors Influencing Rate of a Reaction
Rate of reaction depends upon the experimental conditions such as concentration of reactants (pressure in case of gases), temperature and catalyst.
| Factors | Effect on Reaction Rate |
|---|---|
| 1. Concentration of reactant | The rate of reaction increases with increase in concentration of reactants. |
| 2. Temperature of system | In general, rate of reaction doubles with 10⁰ rise in temperature. |
| 3. Nature of reactant and products | Rate of reaction depends upon the nature of reactants and products. |
| 4. Surface area of reactants | Larger is the surface area of reactants, higher is the rate of reaction. |
| 5. Presence of catalyst | A catalyst increases the rate of reaction. |
| 6. Presence of light | Some photochemical reactions become faster in the presence of light. |
12th Chemistry Unit 3 Multiple Choice Questions
On increasing the temperature of the reacting system by 10° the rate of reaction almost becomes double. The most appropriate reason for this is
Which among the following is a false statement?
The dimensions of rate constant of 2nd order reaction involves
Which of the following statements about the catalyst is true?
Order of a Reaction
the sum of powers of the concentration of the reactants in the rate law expression is called the order of that chemical reaction. Reactions can be first order reaction, second order reaction, pseudo first order reaction etc. depending on the concentration of the reactants.
Zero Order Reactions
Zero order reaction means that the rate of the reaction is proportional to zero power of the concentration of reactants.
The Half-life of a Reaction
The half-life of a reaction is the time in which the concentration of a reactant is reduced to one half of its initial concentration. It is represented as t1/2.
Class 12 Chemistry Chapter 3 Important Questions
What are elementary and complex reactions?
The reactions taking place in one step are called elementary reactions whereas when a sequence of elementary reactions, called mechanism, gives us the product, it is called complex reaction.
Define rate of a reaction.
The rate of a reaction is the change of concentration in any one of the reactants or products per unit time.
Distinguish between rate expression and rate constant of a reaction.
Rate expression is a mathematical expression which denotes the rate of a reaction in terms of molar concentration of reactants.
Rate constant is the rate of the reaction when the concentration of reactants is unity. It is proportionality constant in the rate law and is independent of initial concentrations of the reactants.